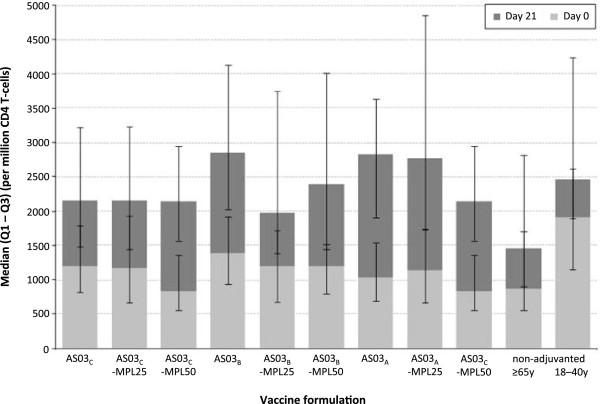
Figure 6.
Cell mediated immunogenicity in the per protocol cell mediated immunogenicity cohort.Footnote: Results for each time point are indicated by median values with first and third quartiles. All participants received inactivated trivalent influenza vaccine, non-adjuvanted (≥65 years and 18–40 years) or formulated with an adjuvant. AS03 is a squalene and α-tocopherol oil-in-water emulsion-based Adjuvant System, with tocopherol content 11.86 mg (A), 5.93 mg (B), or 2.97 mg (C); MPL is 3-O-desacyl-4’- monophosphoryl lipid A: 25 μg (MPL-25) or 50 μg (MPL-50).