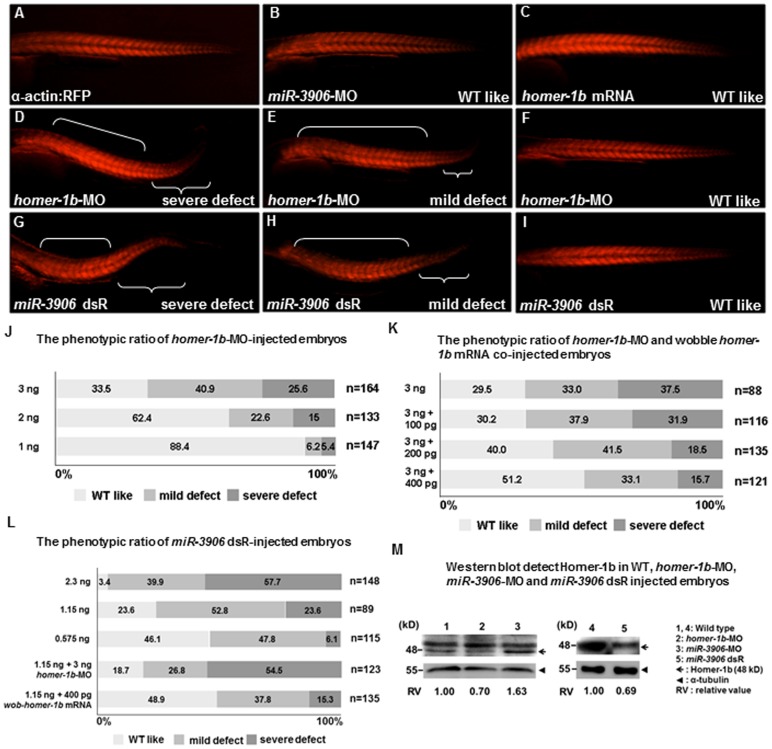
Figure 4. Defective phenotypes induced by injection of excessive miR-3906 and homer-1b-MO were similar.
(A–I) Embryos derived from transgenic line Tg (α-actin:RFP), in which red fluorescence protein (RFP) is expressed specifically in skeletal muscles, were injected with miR-3906-MO, miR-3906 dsR (mature double- strand miR-3906), homer-1b-MO or homer-1b mRNA, as indicated. After injection, the development of trunk muscle was observed at 32 hpf. Compared with WT (A), the morphological trait of embryos injected with either miR-3906-MO (B) or homer-1b mRNA (C) was similar to that of WT. Embryos injected with homer-1b-MO (D–F) and exogenous miR-3906 (miR-3906 dsR) (G–I) displayed such defective phenotypes as body axis bending and trunk muscle shortening in tail. Based on the definition of three defective levels of muscle (see Materials and Methods), we calculated the defective percentages of embryos injected with homer-1b-MO alone (J) and homer-1b-MO plus wobble homer-1b mRNA (K). The occurrence percentages of severe and mild defects of embryos injected with 1.15 ng miR-3906 dsR alone, 1.15 ng miR-3906 dsR plus 3 ng homer-1b-MO and 1.15 ng miR-3906 dsR plus 400 pg homer-1b mRNA were calculated (L). n: indicates the total number of embryos after three injections. (M) Western blot analysis of Homer-1b (48 kD, arrow) in WT (lanes 1 and 4), 3 ng homer-1b-MO-injected embryos (lane 2), 8 ng miR-3906-MO-injected embryos (lane 3) and 1.15 ng miR-3906 dsR-injected embryos (lane 5). Detection of α-tubulin served as internal control (arrowhead). The relative intensity of Homer-1b protein among WT, homer-1b-MO, miR-3906-MO and miR-3906 dsR was also indicated.