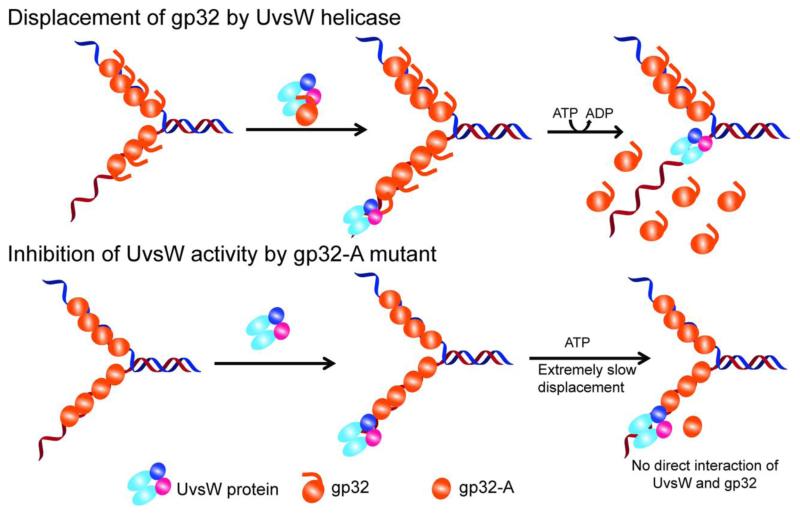
Fig 7.
Schematic representation of the interation of UvsW with gp32 and gp32-A proteins and their effect on the functions of UvsW protein. UvsW binds to gp32 off of DNA and physically interacts with gp32 through the carboxy terminal tail of the ssDNA binding protein. This interaction enhances effective loading of UvsW onto DNA and modulates the various functions of UvsW helicase. UvsW can actively displace gp32 when it encounters them in its path during the various physiological functions during DNA metabolism using the physical interaction of the C-terminal tail of gp32. When the acidic tail is absent as in the gp32-A, their functional interaction is lost and UvsW is unable to displace the protein, and gp32-A merely inhibits the functions of UvsW.