Fig. 1.
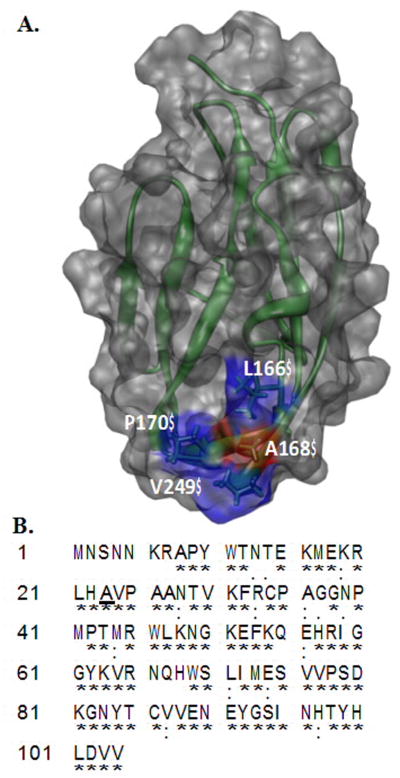
Panel-A, Depiction of the three-dimensional structure of the D2 domain Worldwide Protein Data Bank (PDB ID: 1WVZ) of FGFR2. The residues forming hydrophobic interactions with FGF include A168 (red), L166 (blue), P170 (blue) and V249 (blue). A168 of the D2 domain contacts Tyr-24 and Met- 142 of FGF. Panel-B Amino acid sequence of the D2 domain of FGFR2. The conserved residues in the D2 domain of FGFRs are indicated by an asterix. Ala168 is conserved in FGFRs and is highlighted in bold.