Abstract
Characteristically, most solid tumors exhibit an increased tumor interstitial fluid pressure (TIFP) that directly contributes to the lowered uptake of macromolecular therapeutics into the tumor interstitium. Abnormalities in the tumor-associated lymph vessels are a central brick in the development and prolonged sustaining of an increased TIFP. In the current study, vascular endothelial growth factor C (VEGF-C) was used to enhance tumor-associated lymphangiogenesis as a new mechanism to actively reduce the TIFP by increased lymphatic drainage of the tumor tissue. Human A431 epidermoid vulva carcinoma cells were inoculated in NMRI nu/nu mice to generate a xenograft mouse model. Seven days after tumor cell injection, VEGF-C was peritumorally injected to induce lymphangiogenesis. Tumor growth and TIFP was lowered significantly over time in VEGF-C-treated tumors in comparison to control or VEGF-A-treated animals. These data demonstrate for the first time that actively induced lymphangiogenesis can lower the TIFP in a xenograft tumor model and apparently reduce tumor growth. This model represents a novel approach to modulate biomechanical properties of the tumor interstitium enabling a lowering of TIFP in vivo.
Introduction
Elevated tumor interstitial fluid pressure (TIFP) is a common observation in cancer patients and in experimental tumor models [1–4]. The increased pressure is driven by the abnormal blood and lymphatic vasculature in the tumor tissue that is hallmarked by the highly permeable and chaotic blood vessel network and non-functional lymphatic drainage [5–7]. The enhanced TIFP is the central biophysical protagonist that hampers the enrichment of macromolecular therapeutics within the tumor [8,9].
It has been shown that tumors seem to lack a functional lymphatic vascularization and establish high interstitial fluid pressure values, while in normal tissues excessive fluid is permanently drained by lymphatic vessels [10]. Respectively, it has been reported that in tumors that exhibit signs of lymphatic vessel architecture those were not able to drain tissue fluid. One reason for this phenomenon is the compression of the lymphatic vascular system in tumor tissue by proliferating cancer cells [11]. Furthermore, impairment in the function of the lymphatic valves is under consideration to be another reason why intratumoral lymphatic vessels feature a non-functional state [12]. However, immunohistochemical staining against lymphatic markers often revealed a complete absence of lymph vessels in tumor tissue [7,13]. Nevertheless, no investigations were undertaken so far to investigate how the presence of functional lymphatic vessels in the tumor tissue influences the TIFP in vivo.
We have previously shown that TIFP induces mechanical stretch to tumor cells and contributes as a trigger factor to enhanced tumor cell proliferation [14,15]. Furthermore, we systemically lowered the TIFP and enabled an increased uptake of pharmaceutical active drugs into the tumor tissue [16]. In the latter experimental setup, we applied concentrated human serum albumin as an agent to decrease the TIFP through an enhanced colloid osmotic pressure [16].
In the current work, we are going to address the hypothesis that an increase in functional lymphatics can lower the TIFP through an increased draining of excessive tissue fluid. Subcutaneously, A431 vulva carcinoma-bearing NMRI nude mice were peritumorally treated with vascular endothelial growth factor C (VEGF-C) to enhance lymphangiogenesis in the tumor tissue. VEGF-C is a member of the VEGF family of vascular growth factors, which comprises VEGF-A, -B, -C, and -D and Orf virus VEGFs (or VEGF-E) [17,18]. VEGF-C has been identified to play a crucial role in the development of the lymphatic system [19,20]. In addition, the transgenic overexpression of VEGF-C induced lymphatic vessel enlargement in the skin, and a recombinant VEGF-C approach induced lymphangiogenesis in the chick chorioallantoic membrane [21,22].
In the experimental setup, TIFP and tumor growth were measured and compared with physiologic sodium chloride or VEGF-A-treated xenograft animals. Our findings indicate that the peritumorally applied VEGF-C is able to lower the TIFP and, in addition, delays tumor growth. Immunohistochemical staining of tumor tissue confirmed an increased lymphatic network after peritumoral VEGF-C application.
This work identifies the importance of a functional lymphatic system with regard to increased TIFP featured regularly in solid tumors. It can offer new perspectives for an approach to lower the TIFP through enhanced lymphangiogenesis and simultaneous targeting of tumor tissue with macromolecular therapeutics.
Materials and Methods
Drugs and Cells
The A431 epidermoid vulva carcinoma cell line was purchased from the American Type Culture Collection (ATCC, Manassas, VA) and was cultured in Dulbecco's modified Eagle's medium (Invitrogen, Karlsruhe, Germany) containing 10% fetal calf serum.
VEGF-A (mBA-165, #sc-4571; Santa Cruz Biotechnology, La Jolla, CA) and VEGF-C (#DA3519X; Acris Antibodies, Herford, Germany) were injected peritumorally. Anti-LYVE-1 antibody was obtained from Abcam (Cambridge, United Kingdom; #ab14917) to identify lymphatic endothelial cells. Immunofluorescence visualization was performed using anti-rabbit IgG-fluorescein isothiocyanate secondary antibody (#F0382; Sigma, Steinheim, Germany).
Tumor Models
Female NMRI nu/nu mice (5–6 weeks; 18–22 g; Janvier Elevage, Le Genest-Saint-Isle, France) were subcutaneously injected on both flanks with 5 x 106 A431 cells. All animal experiments were approved in accordance with the German animal welfare regulations (Regierungsprasidium Darmstadt, F79/47). The animals were kept under specific pathogen-free conditions. Sterilized food and tap water were given ad libitum. Mice were anesthetized using isoflurane gas anesthesia (Abbott, Wiesbaden, Germany) for TIFP and tumor growth measurements. For tumor excision, animals were anaesthetized using ketamine/xylazine (100/10 mg/kg, intraperitoneally; Pharmacia, Erlangen, Germany and BayerVital, Leverkusen, Germany). All animals were placed on regulated heating pads to keep the body temperature at 37 to 37.5°C during the experiments. After tumor excision, anesthetized animals were sacrificed.
Tumor size was measured with calipers every 4 days, starting 7 days after subcutaneous tumor inoculation, and tumor volume was calculated as described previously [23,24]. Furthermore, peritumoral injection of VEGF-C (0.5 or 1.0 or 2.0 µg), VEGF-A (1.0 or 2.0 µg), or physiologic sodium chloride (0.9%) was performed 7 days after tumor cell injection.
TIFP Measurements
TIFP was evaluated as reported previously using the “wick-inneedle” technique [14,25,26]. TIFP measurements were done every 4 days using a 27-gauge-sized hollow needle inserted in the outermost tumor tissue layers. The needle was connected to a clinical pressure transducer APT 300 (HSE Harvard Apparatus, March-Hugstetten, Germany). The needle and the adjacent connecting tube were filled with a physiologic sodium chloride solution. Recording of TIFP started when tumors reached a volume of ≥500 mm3. Fluid communication between the needle and the tumor tissue was tested by compressing and decompressing the tubing, and data were accepted only if the recorded pressure returned rapidly to the value before tube compression.
Immunohistochemical Staining
Ki-67 immunohistochemical staining was performed using tumors fixed in 4% paraformaldehyde, dehydrated, and embedded in paraffin. Sectioning was performed using a Leica RM2125 rotary microtome (Leica Microsystems, Wetzlar, Germany). Subsequently, tumor sections were dried for 15 minutes at 65°C, deparaffinized in xylene, rehydrated in series of graded alcohols, rinsed in distilled water followed by heat-induced epitope retrieval in 10 mM citrate buffer (pH 6.0; DakoCytomation, Hamburg, Germany). Then, tissue sections were cooled for 20 minutes, washed [3% H2O2, 0.1% Triton-phosphate-buffered saline (PBS), 2% glycine-PBS], and incubated for 10 minutes with protein blocking agent (Immunotech, Marseille, France). Consecutively, sections were incubated with anti-human Ki-67 (clone MIB-1, 1:50 in PBS; DakoCytomation) for 1 hour at room temperature. After three washes in PBS, sections were incubated with a biotinylated secondary antibody and streptavidin peroxidase reagent (Immunotech) for 45 minutes. Staining was performed using the AEC peroxidase substrate kit (Vector Laboratories, Burlingame, CA). Finally, sections were counterstained using Mayer's hematoxylin (AppliChem, Darmstadt, Germany).
Immunofluorescence
For immunofluorescence studies of lymphangiogenesis, tumors were embedded in TissueTek-Medium (Jung, Heidelberg, Germany) and immediately frozen. Frozen samples were cut in thin slices of 8 to 12 µm using a Leica Kryostat (Leica Microsystems) and placed on object slides. Slides were fixed using cold acetone (-20°C), incubated with anti-LYVE-1 antibody (1:200) for 2 hours at room temperature, and subsequently incubated with fluorescein isothiocyanate-coupled anti-rabbit antibody (1:100, 1 hour at room temperature). Afterward, slides were washed, mounted, and stored at 4°C in nontransparent boxes to protect fluorescent dyes from bleaching. Sections were examined using a BZ8000 fluorescence microscope (Keyence, Neu- Isenburg, Germany).
Quantification of Apoptotic Tumor Cells
The ApopTag Red In Situ Apoptosis Detection Kit (S7165; Chemicon, Temecula, CA) was used to assess cellular apoptosis in frozen sections of tumor tissue that employs terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate (dUTP) nick end labeling (TUNEL) methodology. Frozen slides were fixed for 10 minutes in 1% paraformaldehyde in PBS, washed with PBS, incubated for 5 minutes in -20°C cold ethanol-acetic acid, and washed with PBS. After quenching of endogenous peroxidase activity, samples were incubated with a terminal deoxynucleotidyl transferase enzyme mix (60 minutes at 37°C in a humidified chamber). This step was followed by the incubation of the slides with anti-digoxigenin conjugate for 30 minutes in a light-protected humidified environment. Finally, slides were washed, counterstained, and embedded. We performed nuclear counterstaining using 4′,6-diamidino-2-phenylindole dihydrochloride (AppliChem).
Results
Lowered Tumor Growth Rate in VEGF-C-Treated Xenograft Mice
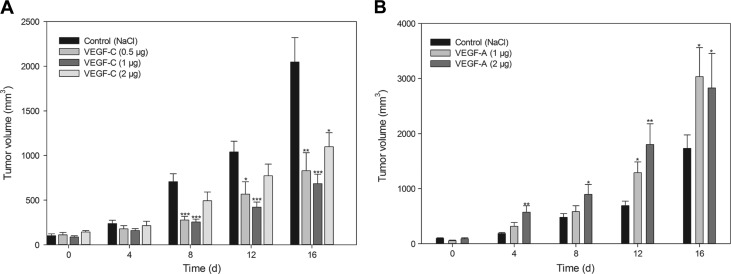
Initially, we recorded the increase of tumor growth in VEGF-C-treated mice versus sodium chloride-treated control animals employing the A431 tumor xenograft model (NMRI nu/nu mice). Therefore, NMRI nude mice were subcutaneously inoculated into the left and right parts of the back with A431 tumor cells. We found that the tumor growth was significantly reduced in all animals treated with VEGF-C that was applied in a peritumoral region 1 week after the inoculation of the tumor cells. Already at 8 days after the VEGF-C application in A431 tumor cells, a significant decrease in tumor growth was visible in the 0.5 (284 ± 26 mm3) and 1.0 µg (258 ± 26 mm3) treated animals in comparison to the control group (707 ± 85 mm3). At 16 days, all three VEGF-C-treated groups (0.5, 1.0, and 2.0 µg) showed a significant reduction in tumor volume (Figure 1A). While control tumors reached a volume of 2051 (±259) mm3, the VEGF-C-treated tumors exhibit tumor volumes of 836 ± 191 mm3 (0.5 µg), 692 ± 90 mm3 (1.0 µg), and 1097 ± 155 mm3 (2.0 µg), respectively.
Figure 1.
Measurement of tumor growth. (A) Peritumoral application of VEGF-C (0.5, 1.0, and 2.0 µg) at day 0. Tumor growth was measured every 4 days using a digital caliper. Data are shown as mean (±SEM) increase of TIFP (n = 10). (B) Peritumoral application of VEGF-A (1.0 and 2.0 µg) at day 0. Tumor growth was measured every 4 days using a digital caliper. Data are shown as mean (±SEM) increase of TIFP (n = 10). For both graphs, *P = .05, **P = .01, and ***P = .001, treatment versus control.
In addition, we tested if the measured effect is specific for VEGF-C or related to the family of VEGFs; we repeated the experiment with VEGF-A, an angiogenesis-related endothelial growth factor. In the new investigation, VEGF-A was applied peritumorally according to the previous VEGF-C experiments. The injections were performed using 1.0 or 2.0 µg of VEGF-A, respectively. In comparison to the previous results of VEGF-C-treated animals, the peritumoral application of VEGF-A significantly increased the tumor growth of the A431 tumors compared to control tissue (Figure 1B).
Tumor Growth Is Decreased due to Lowered Tumor Cell Proliferation
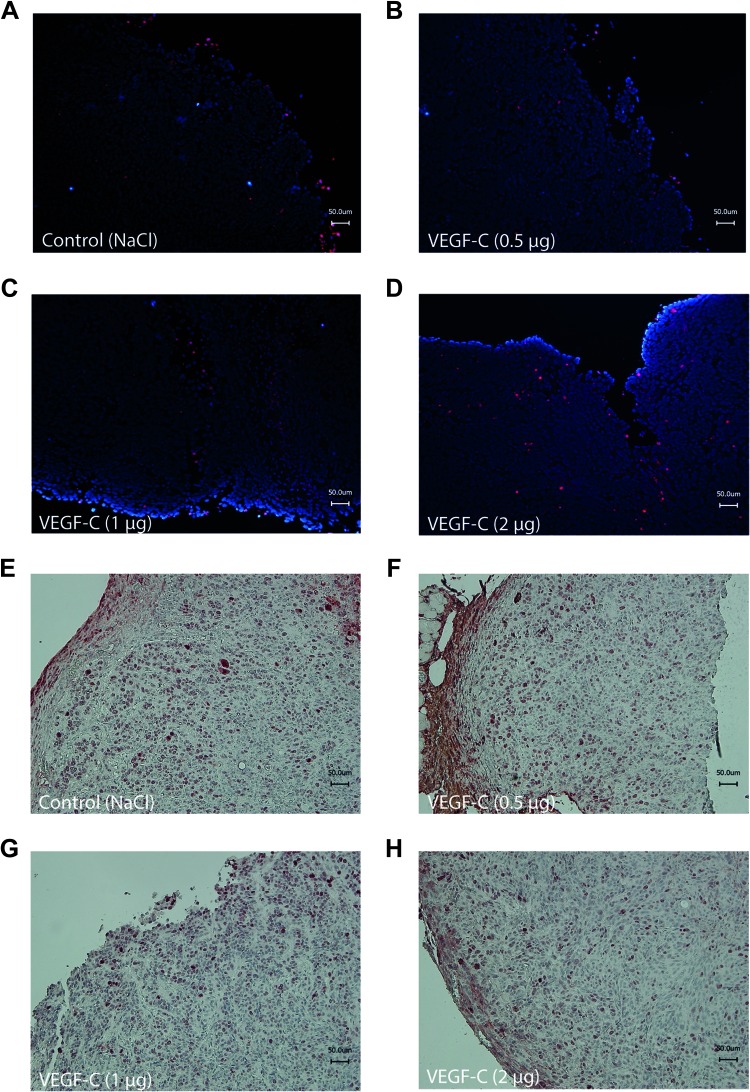
Herein, we investigated if the decelerated tumor growth is due to a reduced tumor cell proliferation or as a result of an increased tumor cell apoptosis. Tumors were excised 7 days after the peritumoral application of VEGF-C to assess cellular apoptosis and cellular proliferation by TUNEL staining or Ki-67 immunohistochemical staining, respectively. Figure 2, A to D, visualizes the results of TUNEL staining for tissue samples of VEGF-C-treated and control tumors. All slides show a similar pattern and number of apoptotic tumor cells. No significant changes are visible between the groups. In contrast, immunohistochemical staining against the cellular proliferation marker Ki-67 revealed a reduced staining in the VEGF-C-treated tumor tissue (Figure 2, F–H) compared to untreated control tumor tissue (Figure 2E). These findings show that the tumor growth rate in VEGF-C-treated tumors is lowered as a result of a decreased tumor cell proliferation, while tumor cell apoptosis is not affected by the VEGF-C application.
Figure 2.
VEGF-C-treated tumors exhibit reduced tumor cell growth but no increase in tumor cell apoptosis. Cellular apoptosis was identified after TUNEL staining of frozen tumor tissue section of (A) control animals or (B) 0.5-µg VEGF-C-, (C) 1.0-µg VEGF-C-, or (D) 2.0-µg VEGF-C-treated animals. Apoptotic cells are red labeled. Frozen sections were counterstained using 4′,6-diamidino-2-phenylindole dihydrochloride. Tumor cell proliferation was assessed by immunohistochemical staining of paraffin-embedded tumor tissue section of (E) control animals or (F) 0.5-µg VEGF-C-, (G) 1.0-µg VEGF-C-, or (H) 2.0-µg VEGF-C-treated animals against the cellular proliferation marker Ki-67. Slides were counterstained using Mayer's hematoxylin.
VEGF-C-induced Lymphangiogenesis in A431 Tumors
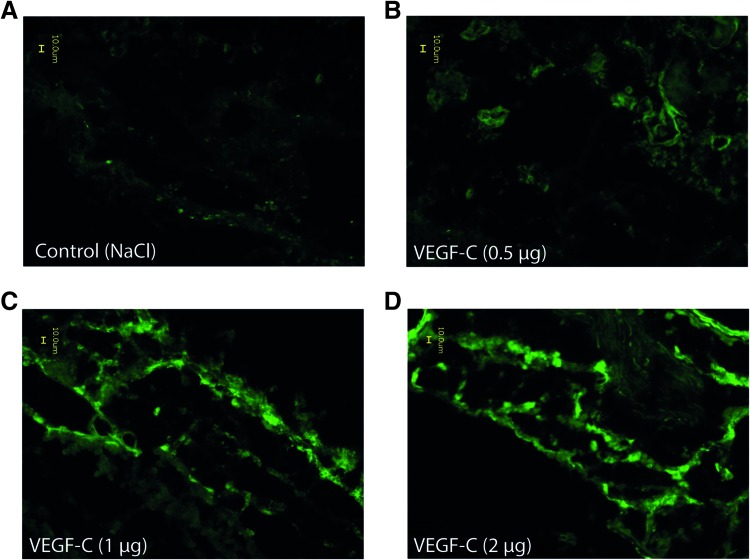
To clarify if the peritumoral application of VEGF-C enhanced lymphangiogenesis in tumor tissue, we excised tumors from all treatment groups at a size of approximately 1000 mm3 and performed immunohistochemical staining with anti-LYVE-1. The antibody binds specific to lymphatic vessel endothelial hyaluronan receptor 1, which is expressed on lymphatic endothelial cells and the hepatic sinusoidal endothelium but not on vascular endothelial cells. Therefore, LYVE-1 staining can be used as a lymphatic endothelial marker [27]. As shown in Figure 3, all VEGF-C-treated tumors highlighted a strong LYVE-1 signal. In comparison to the control tissue that presents only a slight background staining (Figure 3A), tissues of VEGF-C- treated tumors show distinct lymphangiogenesis and lymphatic vessels in the tumor tissue (Figure 3, B–D). Our results also indicated an increased level of lymphangiogenesis in tumors treated with 1.0 or 2.0 µg of VEGF-C compared to tumors that received a peritumoral application of 0.5 µg of VEGF-C. Tumor tissue sections of VEGF-A-treated animals featured no LYVE-1-positive staining (data not shown).
Figure 3.
VEGF-C induced intratumoral lymphangiogenesis in A431 tumors. Immunohistochemical staining of frozen tissue sections of (A) control animals or (B) 0.5-µg VEGF-C-, (C) 1.0-µg VEGF-C-, or (D) 2.0-µg VEGF-C-treated animals against the lymph-specific endothelial marker LYVE-1. Staining was performed on day 12 after the application of VEGF-C.
Reduced Tumor Interstitial Fluid Pressure in VEGF-C-Treated Xenograft Mice
To determine if the VEGF-C-induced lymphatic network influences the TIFP, we used the “wick-in-needle” technique to measure TIFP intratumorally. We started the measurement at day 8 and with a minimum tumor volume of approximately 500 mm3. The TIFP measurement was performed every 4 days. A significant reduction in TIFP is visible for all VEGF-C-treated animals at day 16 in comparison to the sodium chloride-treated control mice (Figure 4). Animals that were previously treated with 1.0 µg of VEGF-C exhibit the highest reduction in TIFP. At day 16, TIFP values of 5.5 (±0.5) mm Hg for 1.0 µg of VEGF-C versus 11.7 (±0.6) mm Hg for the control animals were measured. Furthermore, TIFP values were 8.9 (±1.1) mm Hg for 0.5 µg of VEGF-C and 7.5 (±0.5) mm Hg for 2.0 µg of VEGF-C, respectively. Both values were also significantly lower compared to the control tumors.
Figure 4.
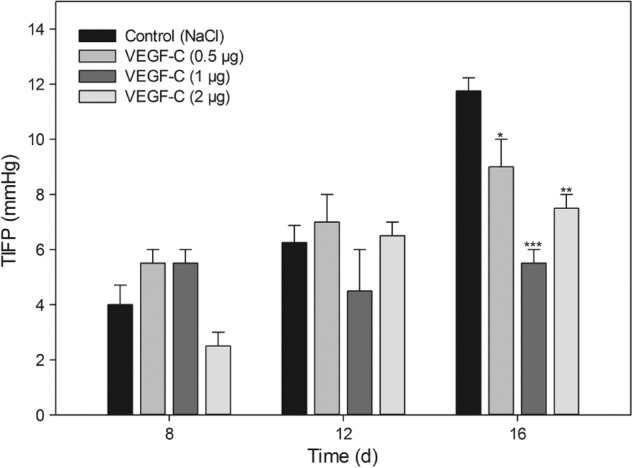
TIFP decrease in VEGF-C-treated animals. TIFP was measured for the first time at day 8 starting at a tumor volume of approximately 500 mm3. TIFP measurements were performed every 4 days consecutively. Data are shown as mean (±SEM) increase of TIFP (n = 4 per group). *P = .05, **P = .01, and ***P = .001, treatment versus control.
Discussion
Elevated TIFP is known to hamper the uptake of macromolecular molecules due to a reduced convectionally driven transendothelial fluid transport [28]. Particularly, the transport and uptake of therapeutic drugs, e.g., monoclonal antibodies, is reduced, contributing to a lowered retention time of chemotherapeutics within tumor tissue [3,29,30]. The lack of a functional lymphatic drainage has been proposed as a key factor in the development of increased values of TIFP in human and experimental tumors [10].
Here, we pursued a new approach to lower the TIFP, highlighting the importance of the lymphatic vasculature in the development and homeostasis of high TIFP values. Initially, VEGF-C was administered peritumoral of vulva carcinoma-derived epithelial A431 tumors that were grown subcutaneously in immunosuppressed NMRI nu/nu mice. As reported previously, A431 tumors exhibit increased TIFP values of up to 15 mm Hg and show a strong correlation between TIFP and tumor volume [14,16]. Respectively, it has been shown by others that TIFP increases with the tumor volume in a number of different tumor species [1,31–33]. In our investigations, we examined the tumor growth in regard to the treatment with different concentrations of VEGF-C. The tumor growth rate was significantly reduced in VEGF-C-treated animals compared with sodium chloride- or VEGF-A-treated mice. Immunohistochemical stainings confirmed that the reduced growth rate of VEGF-C-treated tumors was due to a decreased tumor cell proliferation rate and not apoptosis induced. Hence, we investigated the lymphatic architecture in A431 tumors and found an enhanced lymphatic vascularization in VEGF-C-treated tumors. In addition, a lowered TIFP was measured in mice after being treated peritumorally with VEGF-C. This reduction in TIFP can be explained by the enhanced lymphatic drainage of the tumor interstitium after the VEGF-C application. Furthermore, the investigation of reduced tumor growth is in concert with our previous findings that lowered TIFP reduces tumor growth through mechanical relaxation of the tumor cortex [14,15]. However, studies performed in osteosarcoma cells identified increased VEGF-C levels in tumor cells grown under pressure compared to benign osteoblast values that expressed low VEGF-C levels under the same high pressure cell culture environment [34]. These findings indicate that tumor cells are more sensitive to pressure gradients in interstitial fluid than normal cells. Nevertheless, those findings are preliminary as they have been performed in cell culture and not in tumor models. The current in vivo data do not corroborate an increased lymphatic network in tumors with high TIFP levels in comparison to tumors with low TIFP.
Previous attempts to increase the functional lymphatic vasculature in tumor tissue through overexpression of VEGF-C in tumor cells were capable to induce the formation of functional lymphatics [12]. Nevertheless, those lymphatics were immature and unable to prevent lateral flow into side lymphatics [12]. In our attempt, we induced lymphangiogenesis by applying VEGF-C directly by subcutaneous peritumoral injection. This induced and established intratumoral lymphatic vascularization of the tumor tissue. The significant reduction of TIFP after the administration of VEGF-C can be used as a marker for a functional draining of excessive interstitial fluid. Yet, we cannot affirm that the functionality of the newly developed intratumoral lymphatics equates the drainage capacity of lymphatics outside of the tumor tissue.
In addition, peritumoral application of VEGF-A enhanced tumor growth but did not exhibit a significant effect on the growth of new intratumoral lymphatics in our experimental setup. On a first glance, this finding is in contrary to a range of publications showing that VEGF-A can induce hemangiogenesis and lymphangiogenesis. A closer look reveals that tissue inflammation might play a crucial role in the ability of VEGF-A to induce lymphangiogenesis [35,36]. It is supposed that VEGF-A mediates lymphangiogenesis by binding to the VEGF receptor 1 and recruiting of macrophages, which, in turn, can release additional lymphangiogenic markers [35]. Furthermore, VEGF-A was identified to promote lymphangiogenesis in VEGF-A-overexpressing transgenic animals [37]. However, VEGF-A-transfected H293 could not induce lymphangiogenesis due to lower expression levels of VEGF-A in comparison with transgenic approaches [38]. We assume that a single peritumoral injection as used in our experiment did not provide a VEGF-A level for the induction of lymphangiogenesis.
It has been proven that an increased lymphangiogenesis has a high therapeutic potential in the treatment of lymphedema [39]. Here, we show that using the TIFP-lowering effect of lymphangiogenesis can propose a new approach to increase the uptake of macromolecular therapeutics into tumor tissue. However, it has to be kept in mind that the hypothesized usage of a pro-lymphangiogenic treatment of tumors with the aim of reducing the TIFP has a critical bottleneck that needs to be precluded prior. The active recruitment of functional lymphatic vessels into the tumor can increase the risk of lymphatic tumor cell metastasis. It is known that functional lymphatics in the margin of the tumor surface are sufficient for lymphatic-driven metastasis and intratumoral lymphatic vessels are not necessary for the tumor cells to metastasize [13]. Furthermore, enhanced lymphatic metastasis has been shown previously in VEGF-C-overexpressing tumors indicating that increased VEGF-C levels potentiate the risk of lymphatic metastases significantly [13,40]. During the time course of our experiments, we could not identify an increased risk of metastases in the VEGF-C-treated xenograft animals. Further investigations will have to take place including sentinel lymph node excisions to investigate the risk of increased metastasis due to improved lymphatic drainage of the tumor tissue.
In conclusion, our data show for the first time that the peritumoral application of VEGF-C induces lymphangiogenesis and lowers TIFP in the herein used animal model. Moreover, it was shown that besides lowering of TIFP, VEGF-C-treated tumors also exhibited a delayed growth rate. Thus, the present work displays new insights into the importance of the lymphatic vasculature pertaining to the development and maintenance of high TIFP in solid tumors.
Footnotes
This research was supported by the LOEWE PraBionik network of the state of Hesse (BOSS4 to M.H. and R.P.) and by a Japan Society for the Promotion of Science fellowship to M.H. (PE12081).
References
- 1.Wiig H, Tveit E, Hultborn R, Reed RK, Weiss L. Interstitial fluid pressure in DMBA-induced rat mammary tumours. Scan J Clin Lab Invest. 1982;42:159–164. [PubMed] [Google Scholar]
- 2.Jain RK. Barriers to drug delivery in solid tumors. Sci Am. 1994;271:58–65. doi: 10.1038/scientificamerican0794-58. [DOI] [PubMed] [Google Scholar]
- 3.Jain RK. The next frontier of molecular medicine: delivery of therapeutics. Nat Med. 1998;4:655–657. doi: 10.1038/nm0698-655. [DOI] [PubMed] [Google Scholar]
- 4.Heldin CH, Rubin K, Pietras K, Östman A. High interstitial fluid pressure—an obstacle in cancer therapy. Nat Rev Cancer. 2004;4:806–813. doi: 10.1038/nrc1456. [DOI] [PubMed] [Google Scholar]
- 5.Tufto I, Rofstad EK. Interstitial fluid pressure and capillary diameter distribution in human melanoma xenografts. Microvasc Res. 1999;58:205–214. doi: 10.1006/mvre.1999.2184. [DOI] [PubMed] [Google Scholar]
- 6.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 7.Leu AJ, Berk DA, Lymboussaki A, Alitalo K, Jain RK. Absence of functional lymphatics within a murine sarcoma: a molecular and functional evaluation. Cancer Res. 2000;60:4324–4327. [PubMed] [Google Scholar]
- 8.Jain RK. Transport of molecules in the tumor interstitium: a review. Cancer Res. 1987;47:3039–3051. [PubMed] [Google Scholar]
- 9.Jain RK. Delivery of molecular medicine to solid tumors: lessons from in vivo imaging of gene expression and function. J Control Release. 2001;74:7–25. doi: 10.1016/s0168-3659(01)00306-6. [DOI] [PubMed] [Google Scholar]
- 10.Jain RK, Tong RT, Munn LL. Effect of vascular normalization by antiangiogenic therapy on interstitial hypertension, peritumor edema, and lymphatic metastasis: insights from a mathematical model. Cancer Res. 2007;67:2729–2735. doi: 10.1158/0008-5472.CAN-06-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Padera TP, Stoll BR, Tooredman JB, Capen D, di Tomaso E, Jain RK. Pathology: cancer cells compress intratumour vessels. Nature. 2004;427:695. doi: 10.1038/427695a. [DOI] [PubMed] [Google Scholar]
- 12.Isaka N, Padera TP, Hagendoorn J, Fukumura D, Jain RK. Peritumor lymphatics induced by vascular endothelial growth factor-c exhibit abnormal function. Cancer Res. 2004;64:4400–4404. doi: 10.1158/0008-5472.CAN-04-0752. [DOI] [PubMed] [Google Scholar]
- 13.Padera TP, Kadambi A, di Tomaso E, Carreira CM, Brown EB, Boucher Y, Choi NC, Mathisen D, Wain J, Mark EJ, et al. Lymphatic metastasis in the absence of functional intratumor lymphatics. Science. 2002;296:1883–1886. doi: 10.1126/science.1071420. [DOI] [PubMed] [Google Scholar]
- 14.Hofmann M, Guschel M, Bernd A, Bereiter-Hahn J, Kaufmann R, Tandi C, Wiig H, Kippenberger S. Lowering of tumor interstitial fluid pressure reduces tumor cell proliferation in a xenograft tumor model. Neoplasia. 2006;8:89–95. doi: 10.1593/neo.05469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hofmann M, Schultz M, Bernd A, Bereiter-Hahn J, Kaufmann R, Kippenberger S. Long-term lowering of tumour interstitial fluid pressure reduces Ki-67 expression. J Biomech. 2007;40:2324–2329. doi: 10.1016/j.jbiomech.2006.10.039. [DOI] [PubMed] [Google Scholar]
- 16.Hofmann M, McCormack E, Mujic M, Rossberg M, Bernd A, Bereiter-Hahn J, Gjertsen BT, Wiig H, Kippenberger S. Increased plasma colloid osmotic pressure facilitates the uptake of therapeutic macromolecules in a xenograft tumor model. Neoplasia. 2009;11:812–822. doi: 10.1593/neo.09662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eriksson U, Alitalo K. Structure, expression and receptor-binding properties of novel vascular endothelial growth factors. Curr Top Microbiol Immunol. 1999;237:41–57. doi: 10.1007/978-3-642-59953-8_3. [DOI] [PubMed] [Google Scholar]
- 18.Ferrara N. Vascular endothelial growth factor: molecular and biological aspects. Curr Top Microbiol Immunol. 1999;237:1–30. doi: 10.1007/978-3-642-59953-8_1. [DOI] [PubMed] [Google Scholar]
- 19.Kukk E, Lymboussaki A, Taira S, Kaipainen A, Jeltsch M, Joukov V, Alitalo K. VEGF-C receptor binding and pattern of expression with VEGFR-3 suggests a role in lymphatic vascular development. Development. 1996;122:3829–3837. doi: 10.1242/dev.122.12.3829. [DOI] [PubMed] [Google Scholar]
- 20.Lymboussaki A, Olofsson B, Eriksson U, Alitalo K. Vascular endothelial growth factor (VEGF) and VEGF-C show overlapping binding sites in embryonic endothelia and distinct sites in differentiated adult endothelia. Circ Res. 1999;85:992–999. doi: 10.1161/01.res.85.11.992. [DOI] [PubMed] [Google Scholar]
- 21.Jeltsch MM, Kaipainen A, Joukov V, Meng X, Lakso M, Rauvala H, Swartz M, Fukumura D, Jain RK, Alitalo K. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science. 1997;276:1423–1425. doi: 10.1126/science.276.5317.1423. [DOI] [PubMed] [Google Scholar]
- 22.Oh SJ, Jeltsch MM, Birkenhager R, McCarthy JE, Weich HA, Christ B, Alitalo K, Wilting J. VEGF and VEGF-C: specific induction of angiogenesis and lymphangiogenesis in the differentiated avian chorioallantoic membrane. Dev Biol. 1997;188:96–109. doi: 10.1006/dbio.1997.8639. [DOI] [PubMed] [Google Scholar]
- 23.Baumann M, Appold S, Zimmer J, Scharf M, Beuthien-Baumann B, Dubben HH, Enghardt W, Schreiber A, Eicheler W, Petersen C. Radiobiological hypoxia, oxygen tension, interstitial fluid pressure and relative viable tumour area in two human squamous cell carcinomas in nude mice during fractionated radiotherapy. Acta Oncol. 2001;40(4):519–528. doi: 10.1080/028418601750288262. [DOI] [PubMed] [Google Scholar]
- 24.Obika M, Ogawa H, Takahashi K, Li J, Hatipoglu OF, Cilek MZ, Miyoshi T, Inagaki J, Ohtsuki T, Kusachi S, et al. Tumor growth inhibitory effect of ADAMTS1 is accompanied by the inhibition of tumor angiogenesis. Cancer Sci. 2012;103:1889–1897. doi: 10.1111/j.1349-7006.2012.02381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fadnes HO, Reed RK, Aukland K. Interstitial fluid pressure in rats measured with a modified wick technique. Microvasc Res. 1997;14:27–36. doi: 10.1016/0026-2862(77)90138-8. [DOI] [PubMed] [Google Scholar]
- 26.Pflanzer R, Shelke A, Bereiter-Hahn J, Hofmann M. Ultrasonic quantification of tumor interstitial fluid pressure through scanning acoustic microscopy. In: Nowicki A, Litniewski J, Kujawska T, editors. Acoustical Imaging. Vol. 31. Berlin, Germany: Springer; 2012. pp. 291–299. [Google Scholar]
- 27.Arimoto J, Ikura Y, Suekane T, Nakagawa M, Kitabayashi C, Iwasa Y, Sugioka K, Naruko T, Arakawa T, Ueda M. Expression of LYVE-1 in sinusoidal endothelium is reduced in chronically inflamed human livers. J Gastroenterol. 2010;45:317–325. doi: 10.1007/s00535-009-0152-5. [DOI] [PubMed] [Google Scholar]
- 28.Swabb EA, Wei J, Gullino PM. Diffusion and convection in normal and neoplastic tissues. Cancer Res. 1974;34:2814–2822. [PubMed] [Google Scholar]
- 29.Boucher Y, Jain RK. Microvascular pressure is the principal driving force for interstitial hypertension in solid tumors: implications for vascular collapse. Cancer Res. 1992;52:5110–5114. [PubMed] [Google Scholar]
- 30.Lee I, Boucher Y, Demhartner TJ, Jain RK. Changes in tumour blood flow, oxygenation and interstitial fluid pressure induced by pentoxifylline. Br J Cancer. 1994;69(3):492–496. doi: 10.1038/bjc.1994.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gutmann R, Leunig F, Feyh J, Goetz AE, Messmer K, Kastenbauer E, Jain RK. Interstitial hypertension in head and neck tumors in patients: correlation with tumor size. Cancer Res. 1992;52:1993–1995. [PubMed] [Google Scholar]
- 32.Nathanson SD, Nelson L. Interstitial fluid pressure in breast cancer, benign breast conditions, and breast parenchyma. Ann Surg Oncol. 1994;1:333–338. doi: 10.1007/BF03187139. [DOI] [PubMed] [Google Scholar]
- 33.Stohrer M, Boucher Y, Stangassinger M, Jain RK. Oncotic pressure in solid tumors is elevated. Cancer Res. 2000;60:4251–4255. [PubMed] [Google Scholar]
- 34.Nathan SS, Huvos AG, Casas-Ganem JE, Yang R, Linkov I, Sowers R, DiResta GR, Gorlick R, Healey JH. Tumor interstitial fluid pressure may regulate angiogenic factors in osteosarcoma. J Orthop Res. 2008;26:1520–1525. doi: 10.1002/jor.20633. [DOI] [PubMed] [Google Scholar]
- 35.Cursiefen C, Chen L, Borges LP, Jackson D, Cao J, Radziejewski C, D'Amore PA, Reza Dana M, Wiegand SJ, Streilein JW. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004;113:1040–1050. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Halin C, Tobler NE, Vigl B, Brown LF, Detmar M. VEGF-A produced by chronically inflamed tissue induces lymphangiogenesis in draining lymph nodes. Blood. 2007;110:3158–3167. doi: 10.1182/blood-2007-01-066811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirakawa S, Kodama S, Kunstfeld R, Kajiya K, Brown LF, Detmar M. VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J Exp Med. 2005;201:1089–1099. doi: 10.1084/jem.20041896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stacker SA, Caesar C, Baldwin ME, Thornton GE, William RA, Prevo R, Jackson DG, Nishikawa SI, Kubo H, Achen MG. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med. 2001;7:186–191. doi: 10.1038/84635. [DOI] [PubMed] [Google Scholar]
- 39.Shimizu Y, Shibata R, Shintani S, Ishii M, Murohara T. Therapeutic lymphangiogenesis with implantation of adipose-derived regenerative cells. J Am Heart Assoc. 2012;1:e000877. doi: 10.1161/JAHA.112.000877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mandiota SJ, Jussila L, Jeltsch M, Compagni A, Baetens D, Prevo R, Banerji S, Huarte J, Montesano R, Jackson DG, et al. Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J. 2001;20:671–682. doi: 10.1093/emboj/20.4.672. [DOI] [PMC free article] [PubMed] [Google Scholar]