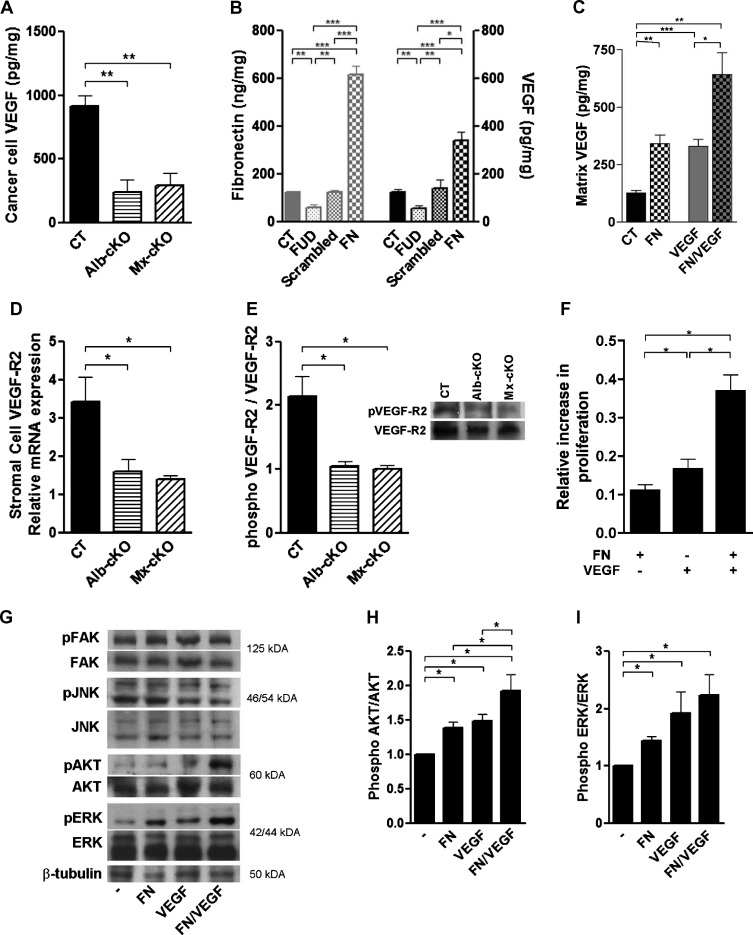
Figure 4.
Mechanistic insights on the reason for decreased angiogenesis. (A) Cancer cell VEGF (by human-specific ELISA) was significantly diminished in tumors originating from cKO mice (N = 4–6/group). (B) A change in matrix fibronectin is associated with a concordant change in VEGF content in the matrix. Cancer cells were cultured in the presence of a peptide (FUD) that inhibits fibronectin deposition or in the presence of 200 µg/ml fibronectin (FN). Matrix was isolated on the basis of deoxycholate (DOC) insolubility, tested for fibronectin and VEGF by ELISA, and adjusted to total protein (N = 4–6/group). (C) Fibronectin retains both endogenous (CT vs FN) and exogenous VEGF (VEGF vs FN/VEGF) in the matrix. MDA cells were cultured for 72 hours in six-well plates without additives, in the presence of 400 µg of fibronectin, 12 ng of VEGF, or both. Matrix VEGF was quantified and corrected to protein in the DOC insoluble fraction as in B (N = 3–4/group). (D) Murine VEGFR-2 mRNA expression was diminished in cKO tumors, but human (Figure W6C) was not (N = 4/group). (E) The amount of phosphorylated VEGFR-2 was also diminished in cKO tumors in densitometry measurements. An example of a Western blot is shown on the right (N = 4/group). (F) Cell proliferation was significantly increased when endothelial cells were treated with either fibronectin or VEGF compared to untreated cells. The addition of both simultaneously resulted in a more pronounced increase in proliferation (N = 3 experiments with three to five replicates/condition per experiment). (G) Both phospho-ERK and phospho-AKT increased 15 minutes after the addition of both fibronectin and VEGF, while phospho-FAK and phospho-JNK did not. (H and I) Densitometries of phospho-AKT and phospho-ERK corrected to total AKT and ERK, respectively. *P < .05, **P < .01, ***P < .0001.