Abstract
As tumors continue to grow and exceed their blood supply, nutrients become limited leading to deficiencies in amino acids (AAD), glucose (GD), and oxygen (hypoxia). These alterations result in significant changes in gene expression. While tumors have been shown to overcome the stress associated with GD or hypoxia by stimulating vascular endothelial growth factor (VEGF)-mediated angiogenesis, the role of AAD in tumor angiogenesis remains to be elucidated. We found that in human tumors, the expression of the general control non-derepressible 2 (GCN2, an AAD sensor) kinase is elevated at both protein and mRNA levels. In vitro studies revealed that VEGF expression is universally induced by AAD treatment in all five cell lines tested (five of five). This is in contrast to two other angiogenesis mediators interleukin-6 (two of five) and fibroblast growth factor 2 (two of five) that have a more restricted expression. Suppressing GCN2 expression significantly decreased AAD-induced VEGF expression. Silencing activating transcription factor 4 (ATF4), a downstream transcription factor of the GCN2 signaling pathway, is also associated with strong inhibition of AAD-induced VEGF expression. PKR-like kinase, the key player in GD-induced unfolded protein response is not involved in this process. In vivo xenograft tumor studies in nonobese diabetic/severe combined immunodeficient mice confirmed that knockdown of GCN2 in tumor cells retards tumor growth and decreases tumor blood vessel density. Our results reveal that the GCN2/ATF4 pathway promotes tumor growth and angiogenesis through AAD-mediated VEGF expression and, thus, is a potential target in cancer therapy.
Introduction
As the blood supply of tumors becomes limited, the demand for oxygen and nutrients such as glucose and amino acids increases accordingly [1–4]. To ensure their survival, tumor cells have developed several strategies to overcome environmental stress. One adaptive mechanism applied by tumors is to reestablish their blood supply by initiating the angiogenic switch. In this setting, proangiogenic mediators are produced with a concomitant reduction of angiogenesis inhibitors [3,5,6].
The eukaryotic translation initiation factor 2α (eIF-2α) can be phosphorylated by four different kinases in response to distinct stressors [7,8]. The ER PKR-like kinase (PERK) and general control nonderepressible 2 (GCN2) are generally activated in the ischemic tumor microenvironment [4,9,10]. ER stress caused by stressors such as glucose deprivation (GD) activates PERK [6,11], while GCN2 is activated by the direct binding of uncharged tRNAs that accumulate during amino acid deprivation (AAD) [8,12,13]. Once activated, GCN2 phosphorylates eIF-2α at serine 51 resulting in reduced protein translation [14–17], whereas translation of several mRNAs including activating transcription factor 4 (ATF4) is upregulated. ATF4 then activates the transcription of genes encoding amino acid biosynthetic enzymes and genes involved in metabolism [18–21].
We and others have shown that ATF4 can regulate VEGF expression by directly binding to its promoter [6,22,23]. We also demonstrated that GD, a common environmental stressor, induces the angiogenic switch in tumors through activation of the PERK/ATF4 pathway, resulting in enhanced tumor angiogenesis [6]. However, studies investigating the role of AAD in tumor angiogenesis are limited and conflicting reports exist regarding the effect of AAD on the production of proangiogenic mediators [3,24]. We hypothesized that as an adaptive response to AAD, tumor cells could increase VEGF production to enhance angiogenesis and in turn sustain tumor growth and progression.
Here, we demonstrate that VEGF expression is markedly increased in response to AAD through activation of the GCN2/ATF4 pathway in vitro. This is supported by the observation that GCN2 knockdown in tumor cells results in impaired tumor growth and angiogenesis in vivo. To our knowledge, there have been no previous reports implicating GCN2 in VEGF regulation and tumor angiogenesis.
Materials and Methods
Cell Lines
The human head and neck squamous cell carcinoma (HNSCC) cell lines UM-SCC-22B, UM-SCC-17B, and UM-SCC-81B (from Dr Thomas E. Carey, Departments of Otorhinolaryngology and Pharmacology, University of Michigan Medical School, Ann Arbor, MI), the breast cancer cell line MCF7 (American Type Culture Collection, Manassas, VA), the glioblastoma cell line U251 (from Dr Yi Sun, Department of Radiation Oncology, University of Michigan, Ann Arbor, MI), and mouse embryonic fibroblast (MEF) cell lines (MEF-PERK+/+ and MEF-PERK-/-, from Dr Andrew Fribley, Department of Pediatrics, Division of Hematology/Oncology, Wayne State University, Detroit, MI) were maintained in Dulbecco's modified eagle's medium (DME) with high glucose (Invitrogen, Carlsbad, CA) and 10% FBS. For AAD studies, amino acid-deficient and control media from Sigma (St Louis, MO) were used (Catalog Nos D9443 and D6046, respectively), and final glucose concentration was adjusted to 25 mM for both media. The AAD medium was also supplemented with sodium pyruvate to match the control medium. All tumor cell lines were authenticated by DNA fingerprinting with small tandem repeat profiling.
Immunohistochemical Analysis
Immunohistochemistry (IHC) was performed as described previously [25]. Antibodies against GCN2 (Cell Signaling Technology, Boston, MA) and CD31 (BD Pharmingen, San Jose, CA) were used. The polink-1 HRP kit with 3-amino-9-ethylcarbazole chromogen was employed for IHC staining. Tumor samples were obtained from the University of Michigan School of Dentistry tissue core; normal human oral mucosa (NHM; from Dr Hector Rios, Department of Periodontics and Oral Medicine, University of Michigan School of Dentistry, Ann Arbor, MI) was used as control. All imaging was done using a Leica DM5000 microscope.
Laser Capture Microdissection
Laser capture microdissection (LCM) was performed as previously described [26]. Approximately 100,000 epithelial cells from either HNSCC or NHM were collected using a pulsed 337-nm UV laser. RNA from at least nine independent tumors and eight NHM tissues were pooled, respectively, and analyzed using real-time quantitative polymerase chain reaction (qPCR).
Immunoblot analysis
Immunoblot analysis was done as previously described [6]. The following primary antibodies were used: ATF4, β-actin (Santa Cruz Biotechnology, Dallas, TX), PERK, and GCN2 (Cell Signaling Technology). HRP-conjugated secondary antibodies were from Santa Cruz Biotechnology. SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific, Rockford, IL) was used to visualize immunoreactive bands.
Enzyme-Linked Immunosorbent Assay
ELISA was performed according to the manufacturer's instructions. Briefly, cell culture supernatants were diluted 1:8 for VEGF, 1:4 for fibroblast growth factor 2 (FGF2), and 1:40 for interleukin-6 (IL-6) and applied to each well (100 µl), then incubated at room temperature for 2 hours and washed three times. The secondary antibody reaction was performed at room temperature (1 hour). Stabilized chromogen was used for colorimetric reactions. Optical density was measured at 450 nm using a plate reader (Spectra Max M2; Molecular Devices Corporation, Sunnyvale, CA).
Lentivirus Infection
Green fluorescent protein (GFP)-expressing lentiviral constructs expressing short hairpin RNA (shRNA) against GCN2, PERK, and ATF4 were from Open Biosystems (Carlsbad, CA). For infection, 1 x 105 cells were plated onto 6-cm plates, infected with the lentivirus, and screened with puromycin (5 µg/ml) to remove non-infected cells. Established stable cell lines were cultured with 2 µg/ml puromycin.
Real-time PCR
Total mRNA was extracted from cultured cells with EZ tissue/cell total RNA mini kit (EZ BioResearch, St Louis, MO) following manufacturer's instructions. cDNA was synthesized using the Verso cDNA kit (Thermo Scientific). Real-time PCR was performed in 384-well plate with the ABI PRISM 7900HT Sequence Detection System. Primers used for qPCR (18S rRNA, VEGF, and GCN2) were from Applied Biosystems (Carlsbad, CA).
Tumor Growth and Angiogenesis In Vivo
Tumor cells (UM-SCC-22B-scshRNA and UM-SCC-22B-shGCN2, 5 x 106) were injected subcutaneously in the flanks of nonobese diabetic/severe combined immunodeficient (SCID) mice (Harlan Laboratories, Haslett, MI). Tumor volume was measured with a digital caliper every 2 days from day 10 post-injection. Tumor volumes were calculated using the formula volume (mm3) = length x width2/2. At the end point, tumors were surgically removed and measured, and angiogenesis was quantitated microscopically. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the University Committee on Use and Care of Animals (UCUCA) of the University of Michigan (Animal Protocol No. 10283-1).
Statistical Analysis
Data are expressed as means ± SD and analyzed using unpaired two-tailed Students' t test. A value of P < .05 was considered to be significant.
Results
Expression of GCN2 Is Elevated in Human Tumors
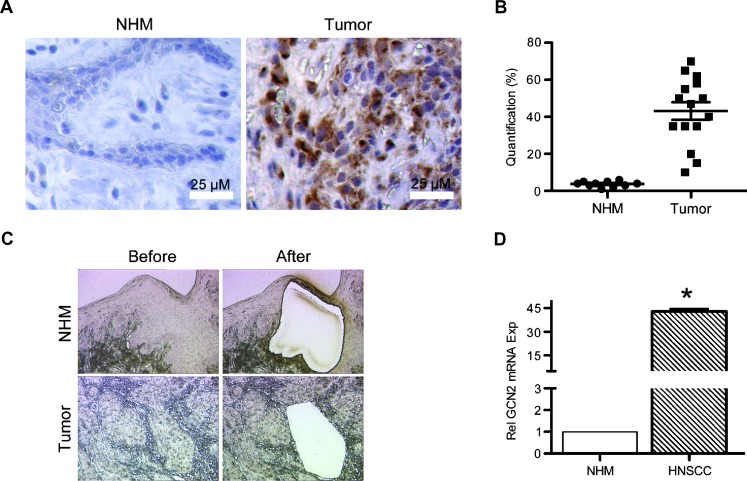
Tumor cells are frequently subjected to glucose and AAD [3]. As a result, metabolic adaptation is required to cope with episodes of nutrient deprivation. GCN2, a sensor of AAD, plays a key role in yeast and mammals in modulating amino acid metabolism in response to nutrient deprivation [27]. It is well established that tumor cells respond to GD by activating the unfolded protein response (UPR), which in turn modulates tumor angiogenesis by activating the angiogenic switch [6]. In HNSCCs, we found that GCN2 expression was significantly increased (43.1%) compared to expression levels in normal human mucosa (NHM, 3.7%; Figure 1A), suggesting a role for GCN2 in tumorigenesis. Using LCM, epithelial cells from both HNSCC (nine patients) and NHM (eight controls; Figure 1C) were collected, and qPCR was carried out to determine relative expression levels of GCN2. A significant increase in GCN2 mRNA was observed in HNSCC (Figure 1D), indicating AAD-induced tumor stress. We have recently reported that GD is associated with increase of the two proangiogenic mediators, IL-6 and VEGF. However, in human tumors where GD and AAD occur simultaneously, the extent to which ADD contributes to GCN2-mediated expression of these mediators remains to be determined.
Figure 1.
GCN2 is upregulated in human oral squamous cell carcinoma. (A) IHC staining of GCN2 in human oral squamous cell carcinoma tissue and normal human mucosa. (B) Quantification showing percentage of GCN2-positive cells. (C) Epithelial cells were collected using LCM from NHM (eight samples, pooled) and HNSCC (nine samples, pooled). (D) qPCR was used to analyze the expression of GCN2. Gene expression levels in tumor tissues were normalized to their expression in normal mucosa (defined as 1). All photos were taken at x 100 magnification. Scale bar, 25 µm. *P < .05.
AAD Universally Induces VEGF Expression in Human Tumors of Different Lineages
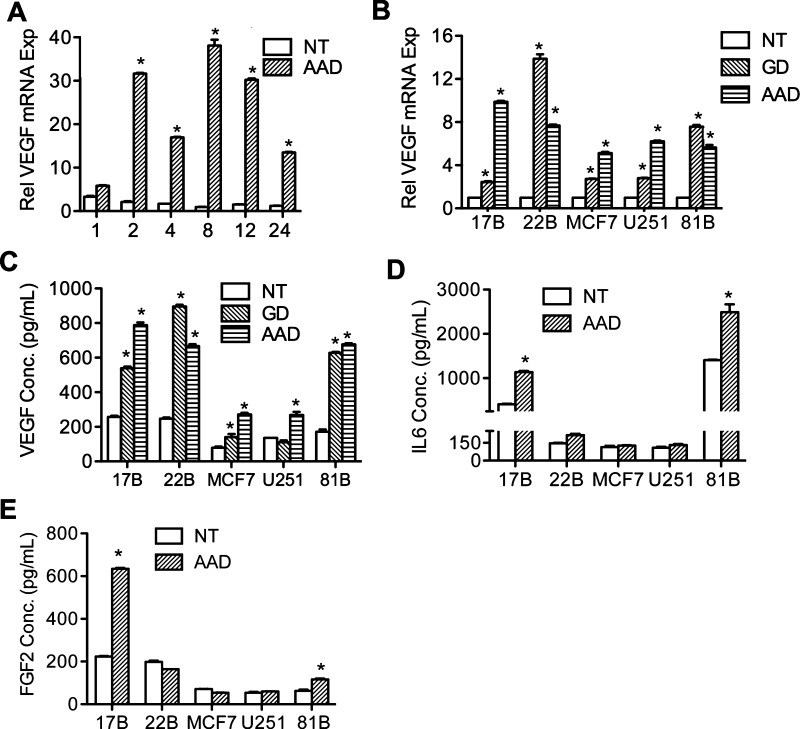
Figure 1 shows that GCN2 is upregulated in HNSCC. We have previously shown that the proangiogenic mediators, IL-6 and VEGF, are increased in human tumors and that UPR induced by GD was responsible for the increased expression of these proangiogenic mediators. Since AAD-induced VEGF mRNA expression has already been reported [3,24], it is possible that AAD can also contribute to expression of these proangiogenic mediators through activation of GCN2. To clarify this relationship, the human oral squamous carcinoma cell line UM-SCC-22B was grown in AAD conditions from 1 to 24 hours. The AAD medium lacks the three amino acids arginine, lysine, and leucine. Final glucose concentration in both the amino acid-deficient and control media was adjusted to 25 mM. Real-time PCR was performed to evaluate VEGF expression. As shown in Figure 2A, AAD induces VEGF expression in a time-dependent manner. To eliminate the possibility of a cell-specific response and to confirm that VEGF expression is AAD-induced, the human glioblastoma cell line U251, the breast cancer cell line MCF7, and two other oral squamous cell carcinoma cell lines UM-SCC-17B and UM-SCC-81B were grown in AAD conditions and VEGF expression was assessed. Tumor cells cultured in GD conditions were used as a positive control. As shown in Figure 2B, AAD induced VEGF expression at mRNA level in all of the cell lines tested, indicating that AAD-induced VEGF expression is a universal phenomenon in human tumors.
Figure 2.
AAD induces VEGF expression in multiple human tumors. (A) Time course study of UM-SCC-22B cells treated with AAD medium (1–24 hours). qPCR revealed a time-dependent change of VEGF expression. (B) qPCR to determine VEGF expression in all five cell lines during untreated, amino acid-deprived, or glucose-deprived conditions for 8 hours. (C–E) ELISA results showing VEGF, IL-6, and FGF2 secretion levels for cell lines indicated during AAD or GD (8 hours). *P < .05.
ELISA was performed to assess the production of VEGF in the supernatant of AAD-treated cells (Figure 2C). We found significant increase in secreted VEGF under AAD treatment. Interestingly, the expression of VEGF induced by GD or AAD was comparable (Figure 2, B and C). Additionally, we investigated the effect of AAD on the expression of two other well-known proangiogenic mediators IL-6 (Figure 2D) and FGF2 (Figure 2E) in different cancer cell lines. As seen, the response of IL-6 and FGF2 to AAD appears to be cell line-specific. We therefore focus on AAD-induced VEGF expression throughout this study. Collectively, these data indicate that AAD plays an important role in differential regulation of angiogenesis mediators in tumor cells regardless of their origin.
AAD-induced VEGF Expression Is Regulated by GCN2
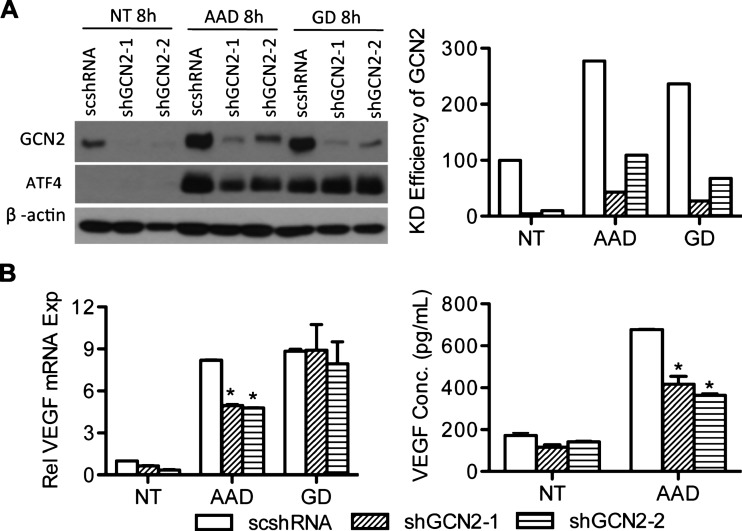
Given that GCN2 is a sensor of AAD, its expression is increased in tumors, and that AAD has been linked to the up-regulation of VEGF, it is plausible that GCN2 contributes to AAD-induced VEGF expression. To test this hypothesis, we used shRNA to suppress GCN2 in UM-SCC-22B cells and established two stable cell lines UM-SCC-22B-shGCN2-1 and UM-SCC-22B-shGCN2-2. High levels of knockdown efficiency were achieved for both shGCN2-1 (95%) and shGCN2-2 (90%). Under AAD treatment, GCN2 is upregulated and phosphorylated (upward shift of bands), but the increase of GCN2 in GCN2 knockdown cell lines is minimal (Figure 3A). Knocking down GCN2 significantly inhibits the expression of VEGF induced by AAD (Figure 3B). These results indicate that GCN2 is essential for AAD-mediated VEGF expression.
Figure 3.
GCN2 plays a key role in AAD-induced VEGF expression. (A) UM-SCC-22B cells were infected with shRNA against GCN2 (shGCN2-1 and shGCN2-2) or scrambled shRNA (scshRNA) and treated with AAD or GD for 8 hours. GCN2 and ATF4 were detected by Western blot (left panel). Percentage of GCN2 knockdown efficiency was quantified with ImageJ (right panel). (B) VEGF mRNA levels were determined using qPCR (left). VEGF secretion was quantified with ELISA (right). *P < .05.
AAD-induced VEGF Expression Is PERK-Independent
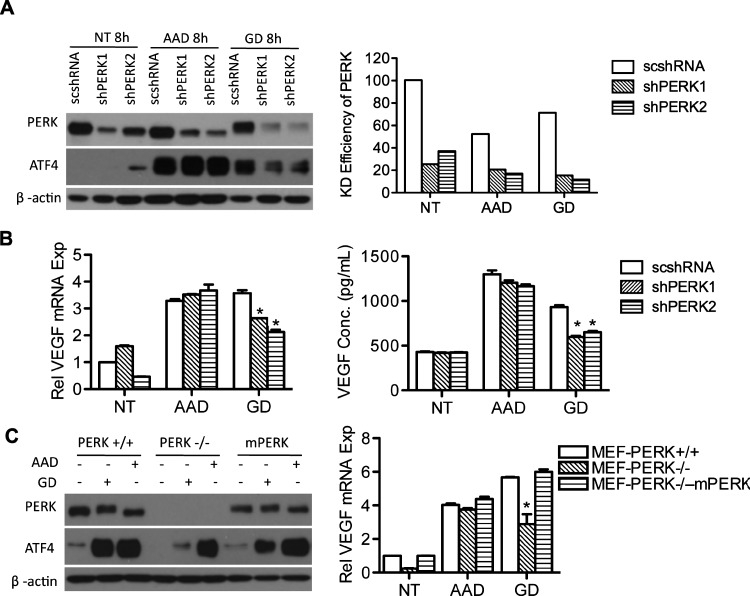
Previously, we have shown that the PERK pathway of the UPR is activated under conditions of GD and regulates VEGF expression through ATF4 [6]. Here, we found that AAD is able to promote the expression of both ATF4 and spliced XBP1, suggesting a possible activation of UPR (data not shown). We therefore wanted to investigate whether VEGF up-regulation that occurs with AAD is PERK-dependent. PERK was knocked down in UM-SCC-22B cells using shRNA generating two stable cell lines UM-SCC-22B-shPERK1 and UM-SCC-22B-shPERK2. The established cell lines were grown under conditions of GD or AAD, followed by assessment of their VEGF expression (Figure 4). As shown in Figure 4A, we were able to achieve significant knockdown efficiency. However, PERK knockdown was unable to reduce ATF4 expression induced by AAD. In contrast, knocking down PERK significantly reduced ATF4 expression induced by GD.
Figure 4.
PERK is not involved in AAD-induced VEGF expression. (A) Stable cell lines 22B-scshRNA, 22B-shPERK1, and 22B-shPERK2 were established with lentiviral vectors and treated with AAD or GD for 8 hours. PERK and ATF4 expression was assessed with Western blot (left). Percentage of PERK knockdown efficiency was quantified with ImageJ (right). (B) qPCR was performed to determine VEGF expression in PERK knockdown cells under AAD or GD (left). ELISA shows secreted VEGF levels in the supernatant (right). (C) PERK and ATF4 were analyzed by immunoblot in three cell lines, PERK+/+, PERK-/-, and rescued PERK knockout MEFs (mPERK) treated with AAD or GD (left). qPCR results showing VEGF transcription levels in all three cell lines (right). *P < .05.
Next, we examined VEGF transcription and secretion during both AAD and GD in PERK knockdown cells. As seen in Figure 4B, both VEGF transcription (left panel) and secretion (right panel) remained unchanged after AAD treatment, demonstrating that PERK is not involved in VEGF expression on AAD. As an alternative approach, we also used PERK-/- MEFs along with their wild-type counterparts to study VEGF expression under AAD and GD (Figure 4C). Similar to our observations in UM-SCC-22B cells, PERK-/- MEFs were still fully able to upregulate ATF4 under AAD, further demonstrating that PERK is not involved in ATF4 regulation during AAD (Figure 4C, left panel). VEGF expression also remained unchanged in PERK-/- MEFs subjected to AAD (Figure 4C, right panel). In contrast, VEGF expression was markedly reduced with GD treatment in PERK-/- MEFs. We also reintroduced mouse PERK (mPERK) in the PERK-/- MEFs and found that ectopic expression of mPERK increased VEGF expression in PERK-/- MEFs during GD but had no effect on AAD-induced VEGF expression (Figure 4C). Collectively, our data exclude the participation of PERK in AAD-mediated VEGF expression.
ATF4 Regulates AAD-Induced VEGF Expression
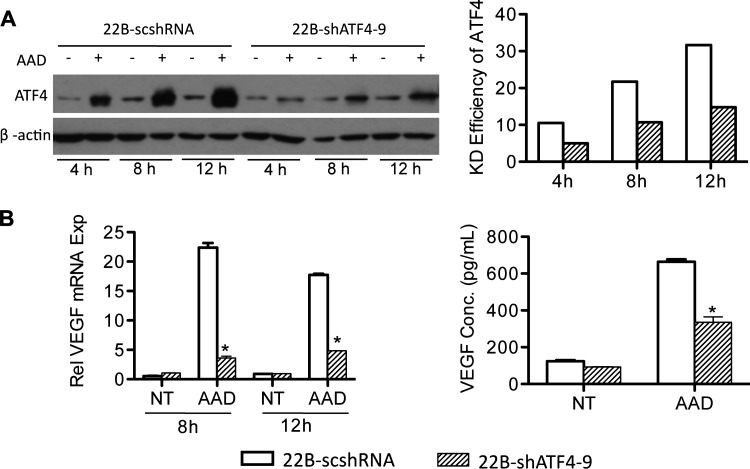
ATF4 regulates VEGF expression by binding to its promoter and is also a common downstream target where the PERK and GCN2 pathways converge [6,22,23]. We found that suppressing GCN2 was able to inhibit AAD-induced ATF4 expression (Figure 3A). To confirm the role of ATF4 in VEGF expression induced by AAD, we knocked down ATF4 in UM-SCC-22B cells using lentiviral constructs and performed a time course analysis. Immunoblots demonstrated a potent knockdown of ATF4 compared to the scrambled control (Figure 5A). We next investigated the effect of ATF4 knockdown on VEGF expression and secretion. As shown in Figure 5B, the knockdown of ATF4 significantly inhibited VEGF expression at both mRNA and protein levels demonstrating a critical role for ATF4 in regulating AAD-mediated VEGF expression.
Figure 5.
ATF4 is involved in AAD-induced VEGF expression. Stable cell lines 22B-scshRNA and 22B-shATF4-9 were established with lentiviral vectors and treated without (NT) or with AAD for 4, 8, and 12 hours. (A) ATF4 expression was assessed with Western blot (left) showing a time-dependent trend. The relative density of ATF4 bands were measured and normalized to NT for all three time points (right). (B) VEGF expression was quantified using qPCR for the 8- and 12-hour time points (left). (C) Secreted VEGF was measured using ELISA for the 12-hour time point. *P < .05.
Inhibition of GCN2 Is Able to Inhibit Tumor Growth and Blood Vessel Formation in a Xenograft Tumor Model
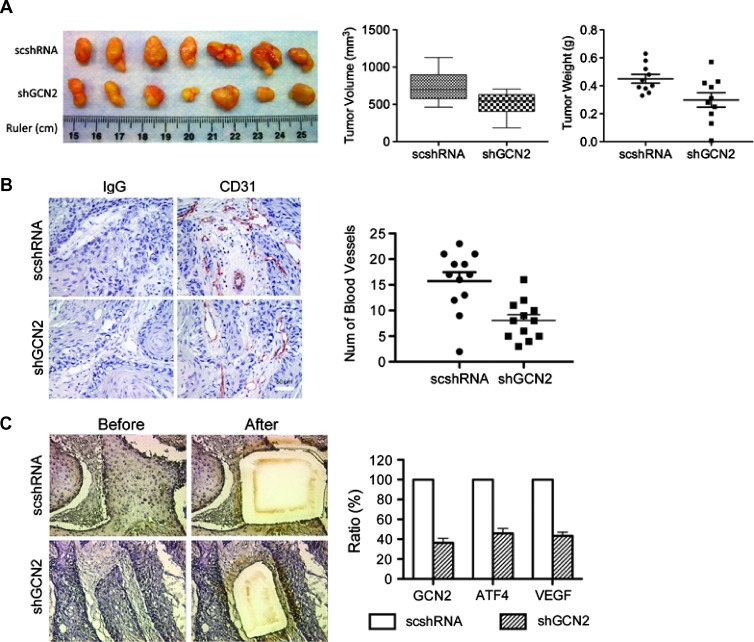
The role of GCN2 has been reported in tumor cell survival and proliferation [4]. However, a role for GCN2 in angiogenesis has not been reported. Investigating effects of GCN2 knockdown in vivo using tumor xenografts in SCID mice can shed light on how GCN2 regulates tumor angiogenesis and growth. UM-SCC-22B-shGCN2-1 cells were injected subcutaneously into SCID mice flanks. UM-SCC-22B-scshRNA cells were used as controls. Thirty days later, tumors were harvested, photographed, and measured. As shown in Figure 6, A and B, GCN2 knockdown in tumor cells significantly reduced tumor volume and weight compared to the controls (P < .05). Blood vessels were stained using CD31 antibody, and random photographs were used for quantification. Blood vessel density was defined as number of blood vessels per field. The results demonstrated a marked decrease of blood vessel density in GCN2 knockdown tumors. To further confirm that GCN2 pathway is functionally impaired in our xenograft model, tumor cells were collected using LCM and subjected to qPCR analysis. The results show that GCN2 was efficiently knocked down, and in turn, its downstream transcription factor ATF4 was inhibited. VEGF expression level was also suppressed (Figure 6C). The results above suggest a critical role for GCN2 in regulating tumor blood vessel growth in vivo.
Figure 6.
Knockdown of GCN2 is sufficient to inhibit tumor growth and blood vessel formation in vivo. Stable cell lines 22B-scshRNA and 22B-shGCN2-1 were injected subcutaneously into flanks of SCID mice. (A) Excised tumors derived from each cell line (left). Tumor volume (middle panel) and tumor weight (right) were measured at the experiment end point. Tumors derived from 22B-shGCN2-1 cells have smaller volumes and lower weight values. (B) Tumor microvessel density was assessed by IHC staining for the endothelial cell marker CD31 (left), and the number of blood vessels per field was quantified (right). IgG was used as negative control. *P < .05. (C) LCM was used to collect tumor cells from xenograft tumor samples and qPCR was used to determine expression levels of GCN2, ATF4, and VEGF.
Discussion
Growing tumors are often subjected to deficiencies in vital nutrients and oxygen. We recently reported that GD contributes to tumor angiogenesis by increasing expression of multiple proangiogenic factors through the PERK/ATF4 pathway [6]. Although AAD-induced VEGF expression has been reported, the results are conflicting. Drogat et al. [24] reported that although glutamine deprivation potently induces VEGF mRNA expression, it leads to the decrease of VEGF expression at the protein level in A549/8 human carcinoma cells. In contrast, Marjon and colleagues found that glutamine deprivation caused a marked induction in both transcription and the secretion of VEGF in a human breast adenocarcinoma cell line (TSE cells) [3]. To elucidate the effect of AAD on VEGF expression, we used tumor cell lines from different lineages, including breast cancer, glioblastoma, and oral squamous cell carcinomas, and studied the effect of AAD on VEGF expression at both mRNA and protein levels. We found that AAD promotes the expression of VEGF in all cell lines tested. The production of VEGF induced by AAD suggests that AAD, a common stressor in the tumor microenvironment, contributes, at least in part, to tumor angiogenesis.
Although the phenomenon of AAD-induced VEGF expression has been described before, the underlying mechanism remains unknown. It is well established that GCN2 is a sensor of AAD and is able to activate ATF4. Our previous work demonstrates that ATF4 is critical in the regulation of proangiogenic mediators. We hypothesized that AAD promotes VEGF expression to accelerate blood vessel formation through GCN2. Indeed, we found that GCN2 is overexpressed in human tumors. In vitro data also showed that AAD could increase GCN2 expression and phosphorylation. shRNA-mediated silencing of GCN2 inhibits AAD-induced ATF4 expression, a downstream transcription factor. Not surprisingly, VEGF expression is significantly inhibited by GCN2 knockdown. To confirm that ATF4 is involved in AAD-mediated VEGF regulation, shRNA against ATF4 was used to reduce its expression. On AAD treatment, VEGF expression was inhibited at both mRNA and protein levels. These results support the hypothesis that AAD induces VEGF expression by activating the GCN2/ATF4 signaling pathway.
We observed that GD was able to promote accumulation of GCN2 but not its phosphorylation (Figure 3). Additionally, we found that GCN2 knockdown does not inhibit GD-induced VEGF expression. It has been reported that when cells experience GD, they use amino acids as a replacement energy supply to ensure their survival [4,10]. Thus, GD appears to contribute to AAD, which in turn activates GCN2. If AAD induces VEGF through GCN2, suppressing GCN2 should be able to inhibit GD-induced VEGF expression. The observed conflict between our studies and the report by Ye and colleagues [4] may be because GD does not cause secondary AAD during early time points and cannot promote the phosphorylation of GCN2. GD-induced VEGF expression may fully depend on the PERK/ATF4 pathway of the UPR at 8 hours of treatment. Therefore, GCN2 does not appear to play a role in GD-induced expression of VEGF in this scenario. It would be interesting to know if GCN2 is involved in GD-induced late-stage VEGF expression.
Jousse and colleagues showed that AAD does not activate the UPR and regulates CHOP expression through a pathway that is independent of the UPR [28]. It has also been reported that GD, and not AAD, activates the UPR in human renal cortical tubular cells in culture [29]. Interestingly, we observed XBP1-s bands in the samples from AAD-treated tumor cells, suggesting possible activation of the UPR by AAD. If so, then PERK should also be activated and be responsible for the increase in ATF4. However, the phosphorylation of PERK was never detected under AAD treatment, and suppressing PERK with shRNA had no effect on AAD-induced VEGF expression, excluding a role of UPR during AAD. It would be interesting to determine how XBP1-s is activated by AAD, considering that that XBP-1s is involved in expression of the inflammatory cytokine IL-6 [30,31] as well as VEGF expression [32].
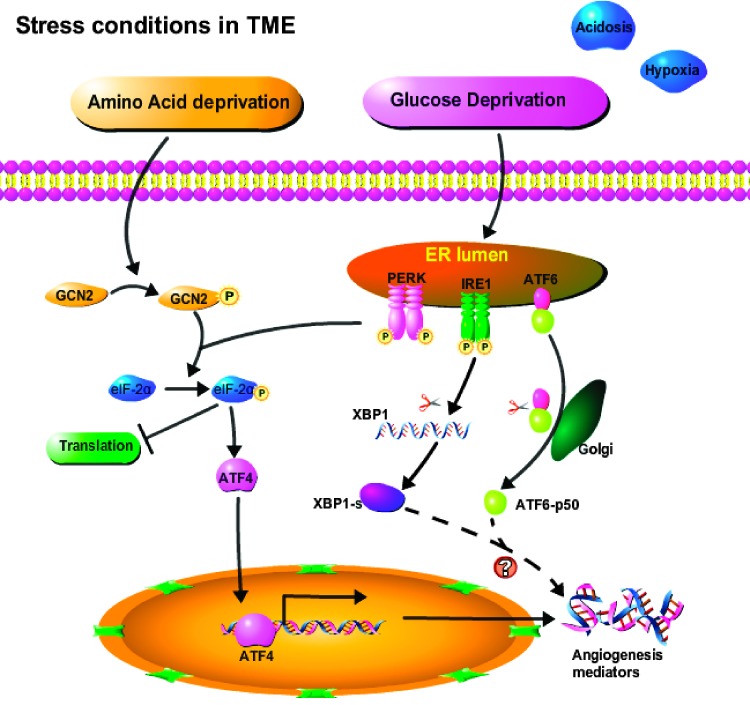
In summary, our studies show that the GCN2/ATF4 pathway is involved in AAD-induced expression of proangiogenic mediators in tumors. Suppressing GCN2 is able to partially inhibit tumor growth and blood vessel formation in xenograft tumors grown in SCID mice. We therefore propose a model (Figure 7) in which the tumor microenvironment (TME) stressors GD and AAD each act through distinct kinases (PERK and GCN2, respectively) to phosphorylate eIF-2α, which in turn leads to reduced global translation with the exception of select genes such as ATF4. Activation of GCN2, PERK, and their downstream target ATF4 is central for tumors to adapt to nutrient deprivation in the TME, eliciting gene expression that enhances tumor angiogenesis, thereby promoting tumor survival and proliferation. Thus, ATF4 appears to be a promising target for antiangiogenic cancer therapy. Moreover, the emerging contribution of environmental stress as a tumor-promoting factor is an important consideration when designing targeted cancer therapies.
Figure 7.
Schematic model demonstrating the mechanisms by which stressors in the tumor microenvironment mediate angiogenesis.
Acknowledgments
We thank members from Jacques E. Nör's laboratory (University of Michigan School of Dentistry, Ann Arbor, MI) and Andrew Fribley (Department of Pediatrics, Division of Hematology/Oncology, Wayne State University, Detroit, MI) for helpful discussions and assistance. We also thank University of Michigan core facilities for their technical assistance.
Abbreviations
- AAD
amino acid deprivation
- ATF4
activating transcription factor 4
- GCN2
general control non-derepressible 2
- GD
glucose deprivation
- HNSCC
human head and neck squamous cell carcinoma
- IL-6
interleukin-6
- LCM
laser capture microdissection
- IHC
immunohistochemistry
- MEF
mouse embryonic fibroblast
- SCID
severe combined immunodeficient
- PERK
PKR-like kinase
- UPR
unfolded protein response
- VEGF
vascular endothelial growth factor
Footnotes
This work was supported by The Sharon and Lauren Daniels Cancer Research Fund, the Office of the Provost, University of Michigan, grant P50-CA97248 from the National Institutes of Health/National Cancer Institute (NIH/NCI), R01-DE21139 from the NIH/National Institute of Dental and Craniofacial Research (NIDCR), and the University of Michigan Cancer Center Support grant (P30 CA046592).
References
- 1.Tsai YC, Weissman AM. The unfolded protein response, degradation from endoplasmic reticulum and cancer. Genes Cancer. 2010;1:764–778. doi: 10.1177/1947601910383011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vaupel P, Hockel M. Blood supply, oxygenation status and metabolic micromilieu of breast cancers: characterization and therapeutic relevance. Int J Oncol. 2000;17:869–879. doi: 10.3892/ijo.17.5.869. [DOI] [PubMed] [Google Scholar]
- 3.Marjon PL, Bobrovnikova-Marjon EV, Abcouwer SF. Expression of the pro-angiogenic factors vascular endothelial growth factor and interleukin-8/CXCL8 by human breast carcinomas is responsive to nutrient deprivation and endoplasmic reticulum stress. Mol Cancer. 2004;3:4. doi: 10.1186/1476-4598-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ye J, Kumanova M, Hart LS, Sloane K, Zhang H, De Panis DN, Bobrovnikova-Marjon E, Diehl JA, Ron D, Koumenis C. The GCN2-ATF4 pathway is critical for tumour cell survival and proliferation in response to nutrient deprivation. EMBO J. 2010;29:2082–2096. doi: 10.1038/emboj.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giordano FJ, Johnson RS. Angiogenesis: the role of the microenvironment in flipping the switch. Curr Opin Genet Dev. 2001;11:35–40. doi: 10.1016/s0959-437x(00)00153-2. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Alam GN, Ning Y, Visioli F, Dong Z, Nor JE, Polverini PJ. The unfolded protein response induces the angiogenic switch in human tumor cells through the PERK/ATF4 pathway. Cancer Res. 2012;72:5396–5406. doi: 10.1158/0008-5472.CAN-12-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tonelli RR, Augusto Lda S, Castilho BA, Schenkman S. Protein synthesis attenuation by phosphorylation of eIF2α is required for the differentiation of Trypanosoma cruzi into infective forms. PLoS One. 2011;6:e27904. doi: 10.1371/journal.pone.0027904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wek RC, Jiang HY, Anthony TG. Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans. 2006;34:7–11. doi: 10.1042/BST20060007. [DOI] [PubMed] [Google Scholar]
- 9.Kumar R, Azam S, Sullivan JM, Owen C, Cavener DR, Zhang P, Ron D, Harding HP, Chen JJ, Han A, et al. Brain ischemia and reperfusion activates the eukaryotic initiation factor 2α kinase, PERK. J Neurochem. 2001;77:1418–1421. doi: 10.1046/j.1471-4159.2001.00387.x. [DOI] [PubMed] [Google Scholar]
- 10.Wek RC, Staschke KA. How do tumours adapt to nutrient stress? EMBO J. 2010;29:1946–1947. doi: 10.1038/emboj.2010.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 12.Dong J, Qiu H, Garcia-Barrio M, Anderson J, Hinnebusch AG. Uncharged tRNA activates GCN2 by displacing the protein kinase moiety from a bipartite tRNA-binding domain. Mol Cell. 2000;6:269–279. doi: 10.1016/s1097-2765(00)00028-9. [DOI] [PubMed] [Google Scholar]
- 13.Hinnebusch AG. Translational regulation of GCN4 and the general amino acid control of yeast. Annu Rev Microbiol. 2005;59:407–450. doi: 10.1146/annurev.micro.59.031805.133833. [DOI] [PubMed] [Google Scholar]
- 14.Krishnamoorthy T, Pavitt GD, Zhang F, Dever TE, Hinnebusch AG. Tight binding of the phosphorylated α subunit of initiation factor 2 (eIF2α) to the regulatory subunits of guanine nucleotide exchange factor eIF2B is required for inhibition of translation initiation. Mol Cell Biol. 2001;21:5018–5030. doi: 10.1128/MCB.21.15.5018-5030.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang P, McGrath BC, Reinert J, Olsen DS, Lei L, Gill S, Wek SA, Vattem KM, Wek RC, Kimball SR, et al. The GCN2 eIF2α kinase is required for adaptation to amino acid deprivation in mice. Mol Cell Biol. 2002;22:6681–6688. doi: 10.1128/MCB.22.19.6681-6688.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jefferson LS, Kimball SR. Amino acid regulation of gene expression. J Nutr. 2001;131:2460S–2466S. doi: 10.1093/jn/131.9.2460S. discussion 2486S-2467S. [DOI] [PubMed] [Google Scholar]
- 17.Kimball SR, Mellor H, Flowers KM, Jefferson LS. Role of translation initiation factor eIF-2B in the regulation of protein synthesis in mammalian cells. Prog Nucleic Acid Res Mol Biol. 1996;54:165–196. doi: 10.1016/s0079-6603(08)60363-3. [DOI] [PubMed] [Google Scholar]
- 18.Lu PD, Harding HP, Ron D. Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J Cell Biol. 2004;167:27–33. doi: 10.1083/jcb.200408003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6:1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 20.Vattem KM, Wek RC. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc Natl Acad Sci USA. 2004;101:11269–11274. doi: 10.1073/pnas.0400541101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siu F, Bain PJ, LeBlanc-Chaffin R, Chen H, Kilberg MS. ATF4 is a mediator of the nutrient-sensing response pathway that activates the human asparagine synthetase gene. J Biol Chem. 2002;277:24120–24127. doi: 10.1074/jbc.M201959200. [DOI] [PubMed] [Google Scholar]
- 22.Roybal CN, Hunsaker LA, Barbash O, Vander Jagt DL, Abcouwer SF. The oxidative stressor arsenite activates vascular endothelial growth factor mRNA transcription by an ATF4-dependent mechanism. J Biol Chem. 2005;280:20331–20339. doi: 10.1074/jbc.M411275200. [DOI] [PubMed] [Google Scholar]
- 23.Oskolkova OV, Afonyushkin T, Leitner A, von Schlieffen E, Gargalovic PS, Lusis AJ, Binder BR, Bochkov VN. ATF4-dependent transcription is a key mechanism in VEGF up-regulation by oxidized phospholipids: critical role of oxidized sn-2 residues in activation of unfolded protein response. Blood. 2008;112:330–339. doi: 10.1182/blood-2007-09-112870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drogat B, Bouchecareilh M, North S, Petibois C, Deleris G, Chevet E, Bikfalvi A, Moenner M. Acute L-glutamine deprivation compromises VEGF-a upregulation in A549/8 human carcinoma cells. J Cell Physiol. 2007;212:463–472. doi: 10.1002/jcp.21044. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Z, Neiva KG, Lingen MW, Ellis LM, Nor JE. VEGF-dependent tumor angiogenesis requires inverse and reciprocal regulation of VEGFR1 and VEGFR2. Cell Death Differ. 2010;17:499–512. doi: 10.1038/cdd.2009.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaneko T, Zhang Z, Mantellini MG, Karl E, Zeitlin B, Verhaegen M, Soengas MS, Lingen M, Strieter RM, Nunez G, et al. Bcl-2 orchestrates a cross-talk between endothelial and tumor cells that promotes tumor growth. Cancer Res. 2007;67:9685–9693. doi: 10.1158/0008-5472.CAN-07-1497. [DOI] [PubMed] [Google Scholar]
- 27.Guo F, Cavener DR. The GCN2 eIF2α kinase regulates fatty-acid homeostasis in the liver during deprivation of an essential amino acid. Cell Metab. 2007;5:103–114. doi: 10.1016/j.cmet.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 28.Jousse C, Bruhat A, Harding HP, Ferrara M, Ron D, Fafournoux P. Amino acid limitation regulates CHOP expression through a specific pathway independent of the unfolded protein response. FEBS Lett. 1999;448:211–216. doi: 10.1016/s0014-5793(99)00373-7. [DOI] [PubMed] [Google Scholar]
- 29.Fougeray S, Bouvier N, Beaune P, Legendre C, Anglicheau D, Thervet E, Pallet N. Metabolic stress promotes renal tubular inflammation by triggering the unfolded protein response. Cell Death Dis. 2011;2:e143. doi: 10.1038/cddis.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwakoshi NN, Lee AH, Vallabhajosyula P, Otipoby KL, Rajewsky K, Glimcher LH. Plasma cell differentiation and the unfolded protein response intersect at the transcription factor XBP-1. Nat Immunol. 2003;4:321–329. doi: 10.1038/ni907. [DOI] [PubMed] [Google Scholar]
- 31.Xu G, Liu K, Anderson J, Patrene K, Lentzsch S, Roodman GD, Ouyang H. Expression of XBP1s in bone marrow stromal cells is critical for myeloma cell growth and osteoclast formation. Blood. 2012;119:4205–4214. doi: 10.1182/blood-2011-05-353300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghosh R, Lipson KL, Sargent KE, Mercurio AM, Hunt JS, Ron D, Urano F. Transcriptional regulation of VEGF-A by the unfolded protein response pathway. PLoS One. 2010;5:e9575. doi: 10.1371/journal.pone.0009575. [DOI] [PMC free article] [PubMed] [Google Scholar]