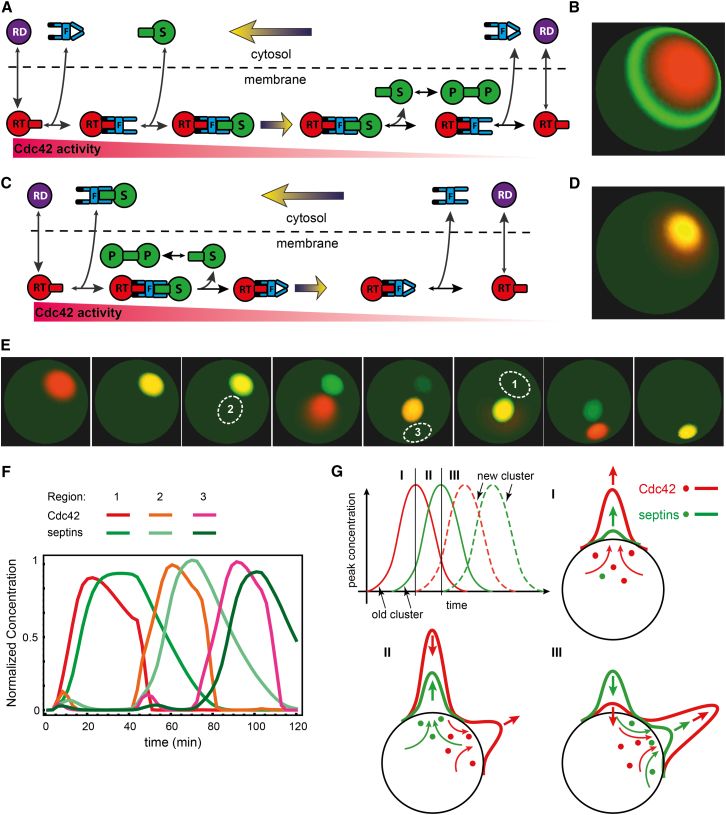
Figure 2.
Model of Effector-Mediated Septin Recruitment Predicts Septin Cap Formation and Explains Chasing
(A) Diagram of the model with exportin-like effector unlocked by Cdc42-GTP to bind septins. S, septin; P, polymeric septins; F, effector; RD, Cdc42-GDP; RT, Cdc42-GTP. Cdc42 activation, RD→RT, in the cluster center (left) and deactivation, RT→RD, on the cluster periphery (right) are shown schematically by arrows. Predominant direction of reactions is indicated by the size of arrowheads. Filled arrows represent diffusion gradients.
(B) Broad septin ring (∼4 μm) forms in the model outlined in (A).
(C) Diagram of the model with importin-like effector that releases septin upon binding to Cdc42-GTP.
(D) Model sketched in (C) predicts a septin cap colocalized with the Cdc42 cluster.
(E) Introduction of septin-mediated negative feedback into the model (C and D) recapitulates chasing phenomenon shown in Figure 1C. See also Movie S3.
(F) Quantification of Cdc42-GTP and septins in the ROIs marked in (E).
(G) Competition for the effector-septin complex explains chasing: (I) Cdc42-GTP recruits septins at the initial location; (II) waning old Cdc42 cluster still recruits septins more efficiently than the new cluster; (III) new cluster fully outcompetes the old.