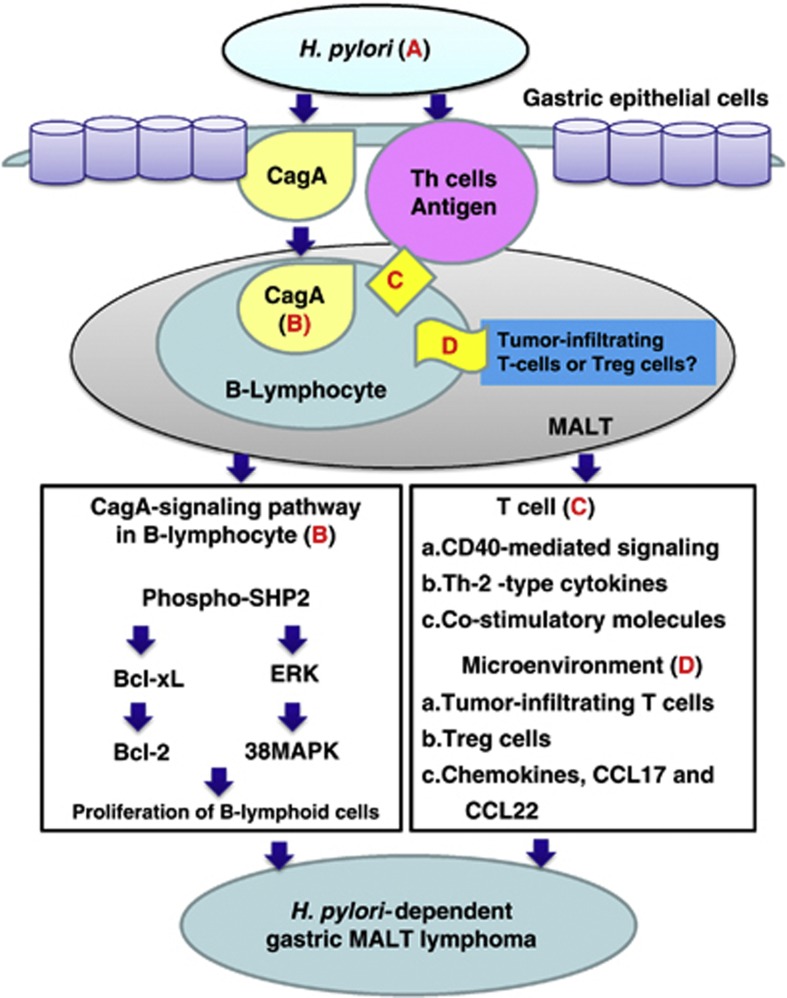
Figure 4.
Involvement of CagA- and T-cell-derived signals in H. pylori-induced lymphomagenesis of gastric MALT lymphoma. (a) Helicobacter pylori (H. pylori) stimulates immune lymphocytes in the gastric mucosa and induces the formation of MALT. B lymphocytes can migrate to and infiltrate the site of H. pylori infection in the stomach. Therefore, H. pylori-specific CagA can be injected into lymphocytes and gastric epithelial cells. (b) The translocated CagA might co-immunoprecipitate with SHP-2 and regulate intracellular signaling pathways, such as by activating extracellular signal-regulated kinase (ERK) and p38 mitogen-activated protein kinase (MAPK) and upregulating Bcl-2 and Bcl-xL expression, leading to B-lymphoid cell proliferation. (c) H. pylori antigenic stimulation, or the triggering of tonic B-cell receptor signaling by the H. pylori antigen, partially drives MALT lymphoma progression. H. pylori can also indirectly promote MALT lymphomagenesis through T-cell stimulation (e.g., CD40-mediated signaling, T helper-2 (Th-2)-type cytokines and costimulatory molecules such as CD86). (d) Molecular crosstalk between B lymphoma cells and tumor microenvironments (tumor-infiltrating T cells, regulatory T-cell cells and chemokines) promotes the survival of B lymphoma cells. Tregs, CD4+CD25+FoxP3+ regulatory T cells.