Fig. 3.
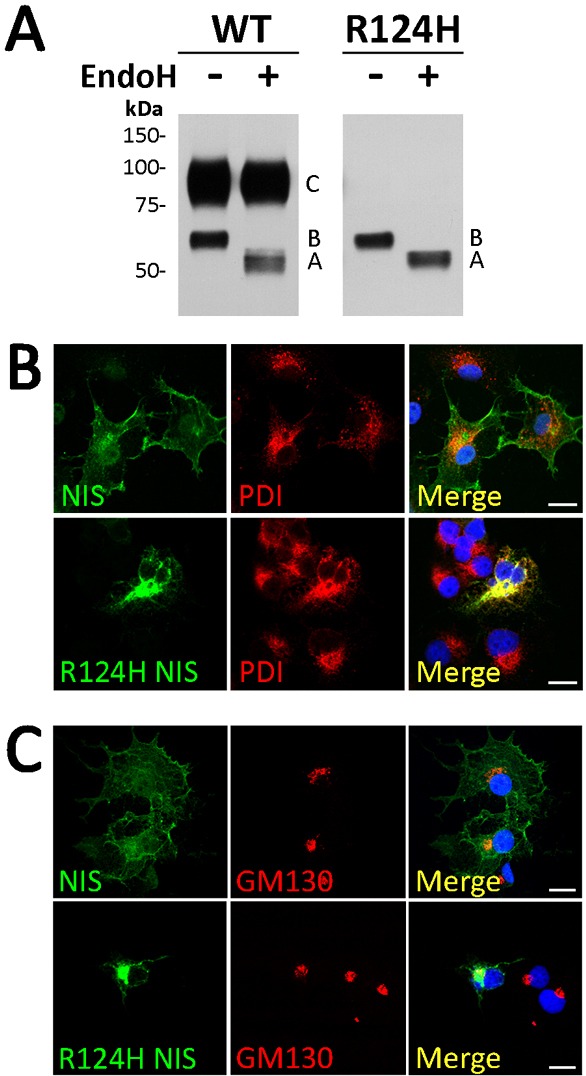
R124H NIS is incompletely glycosylated and intracellularly retained. (A) Immunoblot of membrane fractions treated with Endo H. Membrane proteins (5 µg) from MDCK-II cells permanently expressing WT or R124H NIS were treated (+) or not (−) with Endo H. Samples were resolved by SDS-PAGE and immunoblotted with polyclonal anti-human NIS Ab. Letters on the right side of the blot indicate the relative electrophoretic mobility of the corresponding NIS polypeptides [A: non-glycosylated, B: partially glycosylated, and C: fully glycosylated (mature)]. The blot shown is representative of two independent experiments. (B,C) Immunofluorescence colocalization experiments. Permeabilized WT or R124H NIS-transfected COS-7 cells were incubated with polyclonal anti-human NIS Ab and either anti-protein disulfide isomerase (PDI) or anti-GM130 Abs, followed by Alexa 488- and Alexa 555-conjugated goat anti-rabbit and anti-mouse Abs. Nuclei stained with DRAQ5 are shown in blue. Overlay images (Merge) are shown. Scale bars, 20 µm.
