Abstract
The kidney has a cortico-medullary interstitial gradient of decreasing pH and increasing concentrations of sodium chloride and urea, but the influence of these gradients on receptor signaling is largely unknown. Here, we measured G-protein coupled receptor function in LLC-PK1 cells acutely exposed to conditions mimicking different kidney regions. Signaling through the parathyroid hormone receptor, normally expressed in the cortex, was greatly reduced at an acidic pH similar to that of the inner medulla. Parathyroid hormone receptor, tagged with green fluorescent protein, showed no ligand-induced internalization. In contrast, under both acidic and hyperosmotic conditions, vasopressin increased intracellular cAMP, and upon binding to its type 2 receptor (V2R) was internalized and degraded. Dose-displacement binding assays with selective vasopressin/oxytocin receptor ligands under inner medullary conditions indicated a shift in the V2R pharmacological profile. Oxytocin did not bind to the V2R, as it does under normal conditions and the vasopressin type 1 receptor (V1R) had reduced affinity for vasopressin compared to the V2R in low pH and high osmolality. We suggest that the cortico-medullary gradient causes a receptor-specific selectivity in ligand binding that is of functional significance to the kidney. While the gradient is important for urinary concentration, it may also play a substantial role in fine-tuning of the vasopressin response through the V2R.
Keywords: G-protein-coupled receptor signaling, vasopressin, hypertonicity
The antidiuretic hormone vasopressin (VP) binds to VP receptor type 1 (V1R), VP receptor type 2 (V2R), as well as the oxytocin receptor,1–4 which are all members of the seven membrane spanning G-protein-coupled receptor family. Ligand stimulation of V1R, which is coupled to Gq, leads to an increase in intracellular calcium. In contrast, both V2R and oxytocin receptor are coupled to Gs, and VP binding to these receptors results in an increase in intracellular cAMP. Although activation of these receptors has several effects in the brain and peripheral organs,5–13 the major physiological role of VP is to stimulate water reabsorption in the kidney. VP binding to the V2R, expressed in kidney-collecting duct principal cells, leads to G-protein-induced activation of adenylyl cyclase14–16 and an increase of intracellular cAMP.17 This results in the cell surface accumulation of the AQP2 water channel in principal cells and increased water reabsorption.18–21 After stimulation, VP-bound V2R is desensitized and downregulated,22,23 a process that involves V2R lysosomal degradation.23–25 Binding of VP to the V2R also affects urea and sodium transport and progressively increases concentrations of both sodium and urea in the renal medullary interstitium. This process is important for the generation of the corticopapillary interstitial gradient (Figure 1) that is necessary for urine concentration.26–31
Figure 1. The collecting duct is exposed to a corticomedullary gradient.
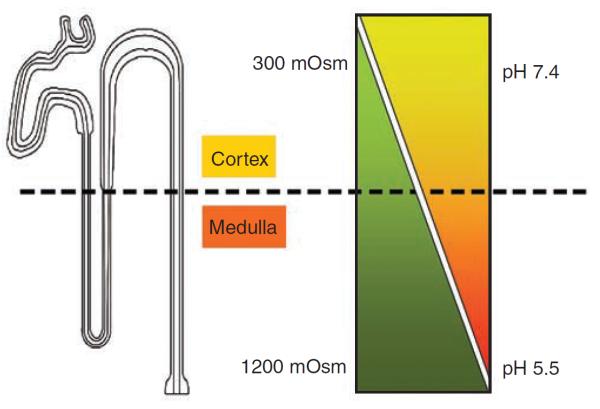
The kidney collecting duct is exposed to a corticomedullary gradient of decreasing pH and increasing concentrations of NaCl and urea.
The V2R is expressed in principal cells of all parts of the kidney-collecting duct, from the cortex to the tip of the papilla32 and the composition of the kidney interstitium is known to vary in pH and osmolarity from cortex to the outer and inner medulla. The cortical interstitium has a neutral pH (7.4) and is isoosmotic (300 mOsm/kg). However, these conditions gradually change along the collecting duct to produce a more extreme environment in which the renal tubules function. At the tip of the papilla, the interstitium is acidic (pH 5.7±0.2)33–35 and hyperosmotic (up to 1200 mOsm/kg in humans and >1200 mOsm/kg in rodents and many other mammals).36–38 Thus, VP must be able to bind to the V2R in environments ranging from isoosmotic/neutral pH to hyperosmotic/acidic pH. The functional requirement of the V2R to be active under the increasingly acidic and hyperosmotic conditions with high urea concentrations that are encountered as the collecting duct descends into the inner medulla, contrasts with that of several other receptors that are present mainly in the renal cortex and outer medulla. These include the parathyroid hormone receptor (PTHR) and the angiotensin II receptor. Interestingly, the V1R, which is present in the renal vasculature, is also found in collecting ducts but is more abundant in the cortex than in the outer or inner medulla.
The role of the medullary environment on VP binding has not been studied in detail. Earlier work from our lab showed that at neutral pH, intracellular cAMP accumulation following VP-induced V2R activation is further increased by hypertoncity.39 cAMP production decreased following addition of urea, but when both sodium chloride (NaCl) and urea were added at gradually increasing concentrations, intracellular cAMP content increased.39 The combined effect of acidification, NaCl and urea, however, remains unknown. Recent work from our group and others has shown that following VP binding at neutral pH, V2R activity is downregulated by lysosomal degradation.24,25 We suggested that this degradation results from the inability of V2R to dissociate from its ligand, VP, in acidic early endosomes. The association between the V2R and VP may have adapted and evolved to allow a tight ligand–receptor interaction that is maintained even under acidic conditions such as those of the inner medulla. It is well established that in contrast to V2R, most ligands detach from their cognate receptor in acidic endosomes before their subsequent lysosomal degradation, whereas the receptor is recycled back to the cell surface.40 Thus, it is important to understand the pharmacology of the V2R under conditions that reflect the prevailing environment of the inner medulla (low pH, high tonicity, and high concentrations of urea) as well as those of other kidney regions.
The purpose of this study was to characterize the pharmacology and downregulation of V2R under conditions mimicking those occurring in the renal cortex and medulla. We show that V2R indeed has properties that allow it to function in the inner medullary environment, in contrast to the PTHR, which is located mostly in the cortex and outer medulla. Our data also show that of all selective VP/oxytocin receptor ligands tested, VP displays the highest affinity for V2R under all conditions tested.
RESULTS
Effects of acidity and hyperosmolality on VP binding to VP receptors
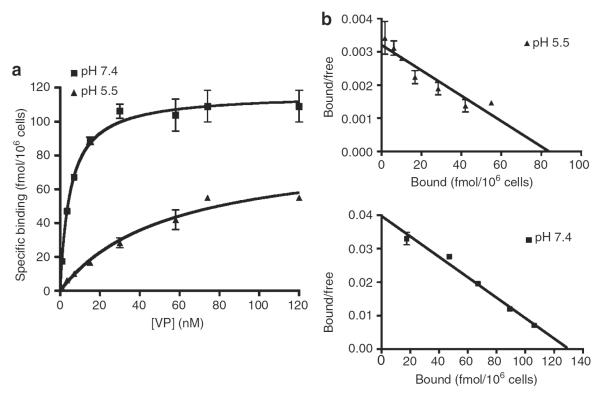
[3H]-VP saturation of binding sites in LLC-FLAG-V2R cells was dose dependent at both pH 7.4 and 5.5 (Figure 2), reaching maximal binding capacity under both conditions (Figure 2a). Scatchard analysis revealed one class of high-affinity binding sites (Figure 2b). The affinity constant (KD) of [3H]-VP for V2R under acidic conditions was 10-fold lower than under conditions of neutral pH (78±34 and 7±3 nm, respectively) but maximal V2R-binding capacity under both conditions was not significantly different (78,000±6000 and 49,800±13,400 binding sites per cell, respectively). A similar result was observed by dose–displacement of [3H]-VP with VP on COS cells transfected with either V1R or V2R (Table 1). Thus, V2R displayed a reduced affinity for VP under acidic conditions, whereas an increase of osmolality had no effect on V2R affinity for VP. In contrast, a combination of acidity and increased osmolality reduced V1R affinity for VP.
Figure 2. Under conditions of acidic pH the affinity of VP for V2R is lower than at neutral pH.
LLC-FLAG-V2R cells were grown to confluence following incubation with different concentrations of [3H]VP at pH 7.4 (■) and pH 5.5 (▲) for 3 h at 4°C (a). Scatchard plot representation of binding saturation experiments at pH 7.4 (■) and pH 5.5 (▲) (b). Means of triplicate values are shown, representative of data from four independent experiments (mean±s.e.m.).
Table 1.
The effect of pH and hypertonicity on VP binding to VIR and V2R
|
IC50 (nm) (n) |
||||
|---|---|---|---|---|
| Receptor | 7.4 | 7.4 NaCI | 5.5 | 5.5 NaCI |
| V1R | 6.0±1.1 (3) | 8.8±1.2 (3) | 7.3±1.8 (3) | 29.3±15.0 (3) |
| V2R | 1.5±0.4 (3) | 2.6±0.6 (3) | 9.1±1.3 (3) | 7.8±2.1 (3) |
VP, vasopressin; V1R and V2R, VP receptor types 1 and 2.
The results shown represent three independent experiments (mean±s.d.).
The effect of an acidic buffer and hyperosmolality on the binding of VP analogs to the V2R was studied by performing [3H]-VP dose–displacement experiments using VP, [deamino-Cys1, D-Arg8]-VP (DDAVP), Manning's compound, and oxytocin at pH 7.4 or 5.5 in the presence or absence of hypertonic NaCl. In all cases, [3H]-VP was displaced in a dose-dependent manner. At neutral pH, V2R affinity for VP was high (KD of 3.67±0.43 nm) (Table 2). V2R affinity for DDAVP was 10-fold lower than for VP whereas its affinity for the Manning's compound and oxytocin was 46- and 179-fold lower than for VP, respectively (Table 2). This pharmacological profile is different from that observed under conditions where either osmolality or pH was modified (VP > Manning's compound > DDAVP). Oxytocin, which has 163 times less affinity for V2R as compared with VP, was unable to displace [3H]-VP from V2R under acidic conditions. This modified pharmacological profile was even more significant when both pH and osmolality were simultaneously changed (Manning's compound > VP > DDAVP).
Table 2.
Affinity of VP analogs for the V2R at pHs 7.4 and 5.5 under isoosmotic and hyperosmotic conditions
| VP analog | KD (nm) at pH 7.4, 300 mOsm | KD (nm) at pH 7.4, 600 mOsm | KD (nm) at pH 5.5, 300 mOsm | KD (nm) at pH 5.5, 600 mOsm |
|---|---|---|---|---|
| VP | 3.67±0.43 (3) | 20.34±9.19 (3) | 88.22±34.08 (3) | 142.40±59.06 (3) |
| DDAVP | 32.21±5.00 (3) | 81.44±44.53 (3) | 600.90±160.71 (3) | 754.92±407.25 (3) |
| Manning's compound | 178.00±54.19 (3) | 42.47±44.53 (3) | 144.40±50.00 (3) | 34.93±12.38 (3) |
| OT | 678.90±192.71 (3) | ND | ⪢ 100,000 (3) | ND |
DDAVP, [deamino-Cys1, d-Arg8]-VP; ND, not defined; OT, oxytocin; VP, vasopressin.
The results shown represent three independent experiments (three) done in triplicate (mean±s.d.).
Effect of acidity and hyperosmolality on V2R downstream effectors and signaling
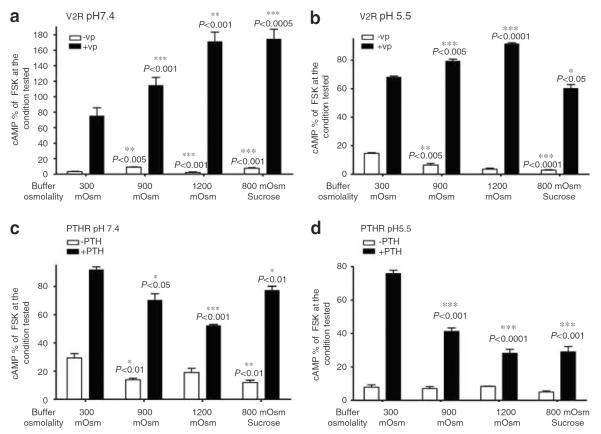
cAMP accumulation induced by forskolin decreased 1.5-fold under acidic conditions, as compared with that induced under conditions of neutral pH (data not shown). Intracellular cAMP was increased by VP stimulation under both normal and acidic conditions, and VP-induced cAMP accumulation was enhanced by tonicity (Figure 3a and b). Similar experiments performed in the presence of sucrose suggest that the effect of NaCl on cAMP is not due to a specific effect of either sodium or chloride. In contrast to the positive effect of hypertonicity on V2R, intracellular cAMP levels in cells expressing PTHR were reduced in hypertonic medium containing PTH under both normal and acidic conditions (Figure 3c or d). Thus, in contrast to VP signaling, PTH signaling was significantly inhibited by hypertonicity (Figure 3c and d). We then investigated the combined effect of NaCl and urea on cAMP generation under conditions of varying pH. For this purpose, we used neutral pH medium mimicking the kidney outer medullary environment and a second medium of pH 5.5, mimicking the kidney inner medulla (Figure 4). We found that in `outer medullary' conditions (600 mOsm/kg, pH 7.4), cAMP generation was increased (Figure 4). Results of the present study (Figure 3a) and our previous study39 show that NaCl added at pH 7.4 increases VP-induced cAMP signaling. We now conclude that 60 mOsm/kg urea does not diminish this effect at a neutral pH. Furthermore, VP causes cAMP content to significantly increase even under conditions mimicking the inner medulla (1200 mOsm/kg, pH 5.5), although this effect was reduced as compared with the signal generated under less extreme `outer medullary' conditions, but did not change in comparison to control conditions (pH 7.4, 300 mOsm/kg).
Figure 3. Hypertonicity increases cAMP signaling of VP-stimulated V2R, but decreases signaling of PTH stimulated PTHR.
cAMP accumulation was tested using a cAMP assay as described in section `Materials and Methods'. cAMP accumulation was studied in the presence or absence of 1 μm VP or PTH (open bars and closed bars, respectively). VP was used to stimulate LLC-FLAG-V2R and PTH was used to stimulate LLC-PTHR cells at pH 7.4 (a, c) or at pH 5.5 (b, d). Experiments were performed in triplicate. The number of experiments performed is shown in parenthesis. Data are shown as mean±s.e.m. Maximal forskolin response at pH 7.4, 300 mOsm for LLC-FLAG-V2R is 11,234±135 fmol/106 cells. Maximal forskolin response at pH 7.4, 300 mOsm for LLC-PTHR is 12,429±491 fmol/106 cells. We compared by t-test the amount of cAMP before VP stimulation at pH 7.4, 300 mOsm to other nonstimulated conditions. Intracellular cAMP accumulation following VP stimulation at pH 7.4, 300 mOsm was compared with VP-induced cAMP accumulation occurring under all other conditions. Significance of t-test is indicated by asterisks.
Figure 4. cAMP accumulation following V2R stimulation by VP occurs under `harsh' conditions mimicking those of the inner medulla.
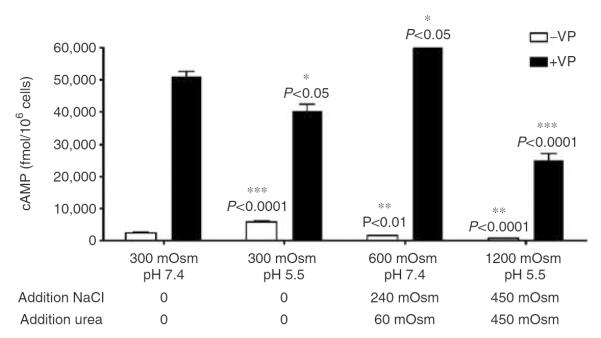
cAMP accumulation was studied in the presence or absence of 1 μm VP (open bars and closed bars, respectively). VP was diluted in normal and acidic buffer in the presence of increasing amounts of sodium and urea. Experiments shown represent an average of three different experiments performed in triplicate (mean±s.e.m.). Maximal forskolin response at pH 7.4, 300 mOsm is 12, 270±467 fmol/106 cells.
Effect of acidity and hyperosmolality on V2R internalization and trafficking
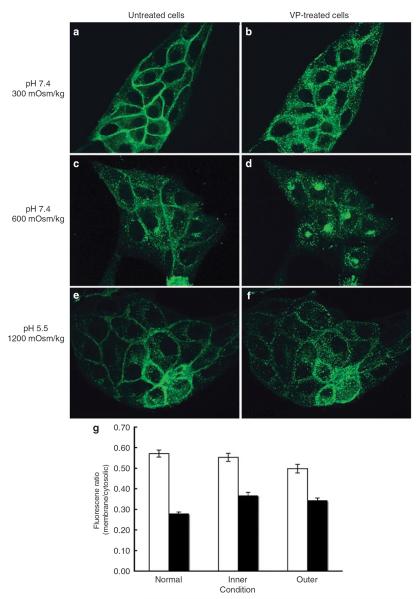
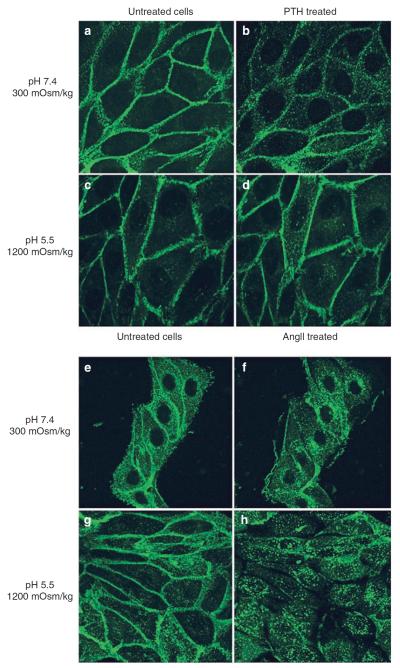
We have shown that even under harsh conditions mimicking the inner medulla, VP significantly increases cAMP generation (Figure 4). To further investigate V2R function, we examined ligand-induced V2R internalization occurring under these conditions. As shown in Figure 5 and confirmed by quantification (Figure 5g), VP induced an internalization of V2R-green fluorescent protein (GFP) under all conditions tested (Figure 5). Similar results were found with two other LLC-PK1 cell lines expressing V2R (data not shown), indicating that this is not a clonal effect. Similar experiments were performed on LLC-PK1 cells stably expressing GFP coupled to either the PTHR (PTHR–GFP) or the angiotensin II receptor (AT1R–GFP) (Figure 6). In the absence of ligands, both receptors were localized at the plasma membrane under normal (Figure 6a and e) or acidic and hyperosmotic conditions (Figure 6c and g). In normal isoosmotic medium at neutral pH, all receptors were internalized in the presence of their respective ligands (Figure 6b and f). In the absence of ligands, an acidic pH together with the addition of both NaCl and urea did not affect the baseline localization of these receptors. However, although addition of PTH did not induce PTHR–GFP internalization under these same conditions, AT1R–GFP was internalized on angiotensin II addition (Figure 6d and h). Quantification of PTHR–GFP fluorescence showed a reduction of membrane fluorescence and an increase of cytoplasmic fluorescence resulting from PTH internalization under conditions mimicking the outer medullary environment. The membrane/cytosolic fluorescence ratio was reduced from 0.56±0.01–0.48 (cortical environment) and 0.38 (outer medullary environment) ±0.01, whereas no significant decrease was observed under conditions mimicking the inner medullary environment. This result suggests that corticomedullary gradients differentially affect receptor internalization. Western blot analysis showed that internalized V2R is degraded under all conditions (Figure 7). Thus, after internalization, V2R is degraded in a similar quantitative manner to that previously reported.24,25
Figure 5. V2R internalizes following VP stimulation under `harsh' conditions mimicking those of the inner medulla.
LLC-V2R-GFP cells were incubated in the absence (a, c, and e) or the presence of 1 μm VP (b, d, and f) under conditions mimicking normal (upper panel), outer (middle panel), and inner medulla (lower panel). In (d), a perinuclear accumulation of V2R was observed after internalization. This phenomenon is currently under more detailed investigation. These results are representative of three independent assays. Three other assays were performed and analyzed by epifluorescence microscopy. Quantification of membrane fluorescence was analyzed using IPLab software (g) as described in section `Materials and Methods'; mean±s.e.m.
Figure 6. AT1R receptors internalize following AngII stimulation under `harsh' inner medullary conditions, whereas PTH-stimulated PTHR does not.
LLC-PTHR-GFP (a–d) and LLC-AT1R-GFP (e–h) cells, stably expressing either PTHR-GFP or AT1R-GFP were incubated in the absence (a, c and e, g) or presence of 1 μm of their respective agonists (b, d and f, h). Cells were incubated with either neutral (a, b and e, f) or acidic hyperosmotic medium (c, d and g, h). Sequential images were acquired using spinning disk confocal microscopy. Acquired images of cells taken before and after 30 min of agonist stimulation are representative of four independent experiments.
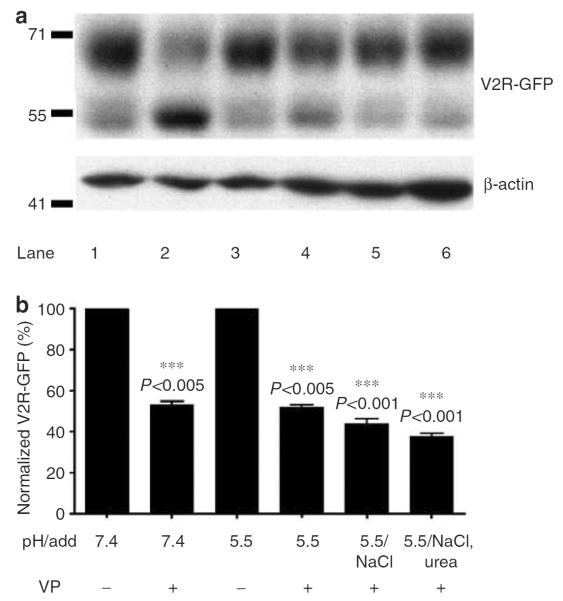
Figure 7. VP stimulated V2R-GFP degrades under conditions of both acidic and neutral pH.
V2R-GFP degradation after 4 hours of VP stimulation (at 37 °C) under both neutral and acidic conditions was studied by Western blot analysis (a). LLC-V2R-GFP cells were incubated in the absence (lanes 1 and 3) or presence (lanes 2 and 4) of VP. In the absence of VP, 71 and 55 kDa bands are observed corresponding to intact, glycosylated V2R and to a degraded fragment of this receptor, respectively. The presence of VP reduced the intensity of the 71 kDa band (lane 2). Similar results were observed under acidic conditions (lanes 4–6). Disappearance of the upper band intensity was quantified in relation to the intensity of corresponding β-actin bands, used as a loading control (b). This is a representative blot from three independent experiments; Mean±SD. ***P>0.005 between VP treated and untreated conditions, ***P>0.001 between sample incubated at pH 5 in the presence of VP and in the presence of either NaCl or Urea.
DISCUSSION
The interstitium of the kidney papilla is a harsh environment in which VP and V2R interact to modify the behavior of specific target cells. Cell signaling becomes a challenge when ligands and their cognate receptors face conditions that are known to affect protein–protein interactions. These conditions include an acidic pH, high concentrations of NaCl, and high concentrations of urea, a compound that affects protein conformation. On the basis of physiological data, it is clear that VP and V2R can indeed interact in such environments, which occur in parts of the kidney medulla. Here, we present data quantifying this interaction under various conditions that partially reflect the in vivo milieu in which V2R ligand stimulation, signaling, and downregulation occur. In this study, we focused our attention on major components of the interstitial environment (NaCl, urea, and pH). However, the presence of other constituents such as reactive oxygen species,41,42 amino acids,43 and organic osmolytes such as betaine, myo-inositol44–46 adds to the complexity of the interstitial environment in which the tubular epithelial cells must function.
First, we studied the effects of environmental acidification on the VP–V2R interaction. We show that VP binds to its receptor and induces an increase of cAMP in an acidic environment, although the affinity of VP for its receptor was reduced under these conditions. A previous study suggested that VP interacts with V2R in LLC-PK1 cells exposed to an even more acidic medium (pH 3).47 This property of VP to bind its receptor under acidic conditions indicates that VP most likely does not detach from its receptor in acidic endosomes (pH 5.5–6.5). We suggest that the `acidic-resistance' of the VP–V2R interaction leads to the delivery of undissociated ligand–receptor complexes to lysosomes where they are degraded, as previously reported by our group and others.48,49
Although acidification of the medium did not significantly affect the affinity of VP for its receptor, it did have interesting effects on the pharmacological profile of the V2R with respect to its affinity for different selective VP/oxytocin receptor ligands. In particular, our data suggest that acidification might be important in discriminating between effects induced by VP and oxytocin in the kidney. Oxytocin moderately increases urine osmolality in VP-deficient Brattleboro rats, which have a reduced capacity to generate a corticomedullary gradient.50–52 Recent data from the Schrier laboratory52 have shown that this occurs in part by modulating AQP2 water channel recycling, supporting our earlier `pre-aquaporin' observations that water channel recycling is stimulated by oxytocin.50–52 Nevertheless, the acute antidiuretic effect of oxytocin was not observed in normal rat whereas its natriuretic effect was observed in normal or VP-deficient rats.53 However, oxytocin given to women to induce labor can be associated with hyponatremia via an effect on the V2R.54,55 Our data suggest that this effect might be restricted to regions of the collecting duct that are not exposed to the harsh interstitial conditions that we show here reduces affinity of the V2R for oxytocin. In this respect, it is important to note that more solute-free water reabsorption occurs in the cortical collecting duct than in the medulla under antidiuretic conditions. On the basis of our present results, environmental acidification has a drastic, negative effect on oxytocin binding. Furthermore, the presence of pH and osmotic gradients may be involved in the fine tuning of VP activation along the collecting duct where V1R and V2R are both present in principal cells.29,56–58 We found that VP interacts differently with the V1R and V2R under acidic and hyperosmotic conditions. Hyperosmotic conditions only slightly reduced the affinity of VP for the V1R and V2R at pH 7.4. Hyperosmotic medium can increase or reduce the affinity of ligands for other receptors as observed for angiotensin and bradykinin, respectively.59 Interestingly, we found that a combination of hyperosmolality and an acidic environment has a much greater effect on reducing VP affinity for the V1R as compared with the V2R. This result suggests that the corticomedullary gradient may be involved in site-specific signaling by regulating (reducing) binding of VP to V1R, a receptor suggested to be a physiological V2R antagonist in parts of the collecting duct.60,61 The gradient might also influence other cross-talk events, such as that occurring between VP and epithelial growth factor, which has been described in rat inner medullary membrane preparations.62
Under normal conditions, we found that the presence of NaCl increases intracellular cAMP levels in response to V2R ligand stimulation. This result is in agreement with previous studies performed on LLC-PK1 cells.39,63,64 Similar results have been observed in isolated rat papillary collecting ducts and mouse thick ascending limbs.32,65,66 Interestingly, although acidification of the cell culture medium reduced ligand affinity and cAMP signaling, simultaneous acidic and hyperosmotic challenge enhanced intracellular cAMP accumulation arising from V2R ligand stimulation. Rescued cAMP signaling by hyperosmolality at acidic pH values was not observed for the PTHR, whose function may, therefore, be impaired under such extreme environments. Indeed the PTHR is largely restricted to the cortex and outer medulla of the kidney.67,68 Thus, in the inner medulla, hyperosmolality is a key factor regulating VP signaling. Although VP significantly increased cAMP concentration under conditions that mimic the harsh environment of the kidney papilla, this accumulation was reduced by urea. A similar dose-dependent reduction has been reported previously in in vitro studies in our lab39 and in previous studies performed on thick ascending limbs of Henle and on kidney medullary tissue.69,70
These results are different to the mutually protective and antiapototic effects of high urea and high salt observed in inner medullary collecting duct cells71,72 but may provide a feedback mechanism that negatively regulates the VP response in vivo by allowing the V2R to monitor, and respond to, interstitial urea accumulation. In addition to examining VP binding and cAMP generation, we examined receptor internalization and found that VP and angiotensin II each induced endocytosis of their specific receptors and that this occurred under all conditions tested. In contrast, PTHR failed to internalize under `inner medullary' conditions. Internalization of AT1R may be expected as it has been shown to modulate VP-induced water transport,73,74 whereas the PTHR is expressed mainly in the cortex and outer medulla and is not exposed to conditions of extreme pH and osmolality.
In summary, we show that VP binds to the V2R and induces cAMP signaling under conditions mimicking the harsh environment of the kidney inner medulla/papilla. Hyperosmolality may rescue the partial loss of affinity of the V2R for its ligand that occurs under acidic conditions. In addition, osmolality and pH can modify the ligand-binding characteristics of V2R. Thus, in addition to its well-known permissive effects on osmotic water transport, the corticomedullary gradient has other positive effects on the urinary concentrating mechanism. These include: (1) increasing AQP2 expression independently of VP,75–77 (2) increasing VP binding and signaling via the V2R in acidic conditions, (3) increasing the efficiency of the VP/V2R association by reducing affinity of the V2R for oxytocin and reducing the affinity of the V1R for VP, and (4) increasing cAMP generation in response to a given concentration of VP. Importantly, the effects vary between different receptors. Although we examined the V2R, PTHR, and some aspects of AT1R function in this report, it is likely that the ligand-binding characteristics and signaling properties of many other receptors in the kidney are modulated by the interstitial environment. This will need to be examined on a case by case basis in future studies.
MATERIALS AND METHODS
Cell culture and transfection
All cell culture reagents were obtained from GIBCO-BRL (Grand Island, NY, USA). LLC-PK1 cells stably expressing either N-terminus FLAG tagged V2R (LLC-FLAG-V2R), C-terminus GFP tagged V2R (LLC-V2R-GFP), or PTHR (HKRK-B7, gift of Dr Gardella, Endocrine Unit MGH) were cultured in DMEM, 10% FBS, neomycin (1 mg/ml) as previously described.22,24,78 COS cells were grown in a similar medium lacking neomycin. COS and LLC-PK1 cells were transfected with vectors containing V1R or V2R (University of Missouri-Rolla, cDNA Resource Center, MO, USA) or angiotensin receptor type 1 conjugated to GFP (AT1R-GFP) (Gift of Dr Guillemette, University of Sherbrooke, Canada). Transfections were carried out with effectene according to Qiagen protocol (Hilden, Germany).
[3H]-VP binding assays
Unless otherwise stated, all chemicals were purchased from Sigma (St Louis, MO, USA). The dose–displacement and saturation binding assays were both performed under either normal or acidic conditions. [3H]-VP binding site saturation was performed under normal conditions as previously described.22 [3H]-VP (44.0 Ci/mmol, Perkin-Elmer, Boston, MA, USA) was diluted in 20 mm sodium phosphate buffer pH 7.4, 1 mg/ml glucose, 1 mm CaCl2, 1 mm MgCl2, 3.5 mm KCl, and 137 mm NaCl. To obtain acidic conditions, sodium phosphate was replaced by 20 mm MES (2-N-morpholino)ethanesulfonic acid) pH 5.5. For both conditions, an increasing amount of [3H]-VP (up to 120 nm) was added to each well, in triplicate for each experiment. Nonspecific binding was determined in the presence of excess unlabeled VP (1 mm). After incubation (3 h, 4 °C), washed cells were solubilized in 500 μl of NaOH (0.1 N). Radioactivity signals were quantified with a liquid scintillation analyzer Tricarb 2200 CA (Packard, The Netherlands).
[3H]-VP (10 nm) was displaced by increasing amounts of [Arg8]-VP (VP), oxytocin, [β-mercapto-β,β-cyclopentamethylenepropionyl1, O-me-Tyr2, Arg8]-VP (Manning's compound), or DDAVP on LLC-FLAG-V2R cells. Each dose–displacement curve was performed under neutral and acidic conditions in buffers of 300–600 mOsm (following addition of NaCl). The nonspecific binding was defined in the presence of VP (1 μm). Dose–displacement of [3H]-VP by VP under similar buffer conditions was also performed on COS cells transfected with V1R or V2R as described above.
cAMP assays
Intracellular cAMP levels were measured with the Biotrak kit in the constant presence of 3-isobutyl-1-methyl-xanthine (IBMX, 1 mm), a phosphodiesterase inhibitor (Amersham Biosciences Corp., Piscatway, NJ, USA) as previously described.22,24 Intracellular cAMP accumulation was evaluated in either LLC-FLAG-V2R or LLCPTHR cells after incubation in the presence of 1 μm VP or PTH, respectively. Cells were incubated in the presence of either VP or PTH diluted in normal or acidic isotonic buffer (300 mOsm) and in normal or acidic hyperosmotic buffer (900 and 1200 mOsm or 30 min at 37 °C. All cAMP assays were performed on quadruplicate where maximal cAMP elevation was evaluated in the presence of forskolin (10 μm). The combined effect of urea and NaCl on VP-induced intracellular cAMP accumulation in LLC-V2R-GFP cells was studied. Cells were incubated in the presence or absence of VP (1 μm) for 30 min in normal or acidic buffer to which urea and NaCl were added (120 mOsm/kg NaCl and 60 mOsm/kg urea, and 225 mOsm/kg NaCl and 450 mOsm/kg urea, respectively).
Spinning disc confocal microscopy
V2R-GFP internalization in the presence of VP in neutral isotonic buffer, and buffers mimicking outer and inner medullary conditions (see section `cAMP assays') was followed in real-time, using spinning disc confocal microscopy (LCI Ultra View, Perkin-Elmer). The microscope was equipped with an environmental chamber to regulate temperature (37 °C) and atmosphere (95% O2/5% CO2). The cell culture medium in the glass-bottomed dishes (WPI, Sarasota, FL, USA) containing cells was replaced by different media as indicated in the figure legends, and cells were placed in the environmental chamber. After 15 min, ligand (VP, PTH, or angiotensin II) was then added at a final concentration of 1 μm. In order to reduce fluorescence bleaching, images of cells were taken before and after 30 min of agonist stimulation. At each time point, stacks of images were taken at 0.2 μm intervals along the z axis, and a three-dimensional image was reconstructed using Volocity software (Improvision Inc., Waltham, MA, USA). In parallel, quantification of fluorescence at the membrane and in intracellular compartments in the presence or absence of ligands was performed on cells fixed in PBS containing 4% paraformaldehyde and 5% sucrose. Quantification was performed using IPlab software (BD Biosciences, CA, USA). Briefly, the number of pixels contained in an outline enclosing the plasma membrane or the cell cytoplasm was quantified as integrated optical density 23 values. The mean plasma membrane/cytoplasm sorting index, defined as the intregated optical density of the plasma membrane divided by the cytoplasmic intregated optical density, ±s.e.m from at least 20 cells was determined.
Electrophoresis and Western blot analysis
The effect of NaCl and urea on V2R degradation was examined by Western blot analysis as already described.24 In brief, LLC-V2R-GFP cells were incubated for 4 h in the presence or absence of VP (1 μm), diluted in either normal or acidic medium containing either NaCl or urea. Protein (20 mg) solubilized in RIPA buffer (Boston Biopro-ducts, MA, USA), 4 mm EDTA, and protease inhibitor cocktail (Roche Diagnostics GmbH, Germany) was separated on 4–12% Bis-Tris-PAGE gel and transferred onto PDVF membranes using the IBlot system according to the manufacturer's protocol (Invitrogen, Carlsbad, CA, USA). The presence of V2R-GFP was detected using a polyclonal rabbit anti-GFP antibody (0.4 μg/ml, Molecular probe, OR, USA). The acid-tripped membranes were incubated with a mouse anti-β-actin antibody (0.2 μg/ml, Chemicon International, Temecula, CA, USA) and used as loading control. All band intensities were quantified using a video densitometer and Kodak ID software (Kodak, New Haven, CT, USA).
Statistic and analysis of binding data
The values of IC50, KD, and Bmax as well as the values of EC50 in cAMP assays were analyzed using a nonlinear least squares fitting program (GraphPad PRISM, GraphPad Software Inc., CA, USA) and expressed as means±s.e.m. Statistical analyses were performed using the unpaired Student's t-test.
ACKNOWLEDGMENTS
This work was supported by NIH grants PO1DK38452 (DB and DAA). H.Y. Lin was supported by an NIH RO1award (DK071837). R. Bouley received an investigator award from the National Kidney Foundation. U. Hasler is supported by a Swiss FSBMB Fellowship as well as an ECOR Fellowship awarded by MGH. The Microscopy Core facility of the MGH Program in Membrane Biology receives additional support from the Boston Area Diabetes and Endocrinology Research Center (DK57521) and the Center for the Study of Inflammatory Bowel Disease (DK43341).
Footnotes
DISCLOSURE All the authors declared no competing interests.
REFERENCES
- 1.Birnbaumer M, Seibold A, Gilbert S, et al. Molecular cloning of the receptor for human antidiuretic hormone. Nature. 1992;357:333–335. doi: 10.1038/357333a0. [DOI] [PubMed] [Google Scholar]
- 2.Lolait SJ, O'Carroll AM, McBride OW, et al. Cloning and characterization of a vasopressin V2 receptor and possible link to nephrogenic diabetes insipidus. Nature. 1992;357:336–339. doi: 10.1038/357336a0. [DOI] [PubMed] [Google Scholar]
- 3.Morel A, O'Carroll AM, Brownstein MJ, et al. Molecular cloning and expression of a rat V1a arginine vasopressin receptor. Nature. 1992;356:523–526. doi: 10.1038/356523a0. [DOI] [PubMed] [Google Scholar]
- 4.Sugimoto T, Saito M, Mochizuki S, et al. Molecular cloning and functional expression of a cDNA encoding the human V1b vasopressin receptor. J Biol Chem. 1994;269:27088–27092. [PubMed] [Google Scholar]
- 5.Calo G, Rizzi A, Traina L, et al. Pharmacological characterization of a vasopressin V1 receptor in the isolated human gastric artery. Life Sci. 1997;60:PL63–PL68. doi: 10.1016/s0024-3205(96)00635-2. [DOI] [PubMed] [Google Scholar]
- 6.Jovanovic A, Grbovic L, Zikic I, et al. Characterization of arginine vasopressin actions in human uterine artery: lack of role of the vascular endothelium. Br J Pharmacol. 1995;115:1295–1301. doi: 10.1111/j.1476-5381.1995.tb15039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loup F, Tribollet E, Dubois-Dauphin M, et al. Localization of high-affinity binding sites for oxytocin and vasopressin in the human brain. An autoradiographic study. Brain Res. 1991;555:220–232. doi: 10.1016/0006-8993(91)90345-v. [DOI] [PubMed] [Google Scholar]
- 8.Saito M, Sugimoto T, Tahara A, et al. Molecular cloning and characterization of rat V1b vasopressin receptor: evidence for its expression in extra-pituitary tissues. Biochem Biophys Res Commun. 1995;212:751–757. doi: 10.1006/bbrc.1995.2033. [DOI] [PubMed] [Google Scholar]
- 9.Serradeil-Le Gal C, Raufaste D, Marty E, et al. Autoradiographic localization of vasopressin V1a receptors in the rat kidney using [3H]-SR 49059. Kidney Int. 1996;50:499–505. doi: 10.1038/ki.1996.341. [DOI] [PubMed] [Google Scholar]
- 10.Tahara A, Tsukada J, Tomura Y, et al. Pharmacologic characterization of the oxytocin receptor in human uterine smooth muscle cells. Br J Pharmacol. 2000;129:131–139. doi: 10.1038/sj.bjp.0702996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tahri-Joutei A, Fillion C, Bedin M, et al. Local control of Leydig cell arginine vasopressin receptor by naloxone. Mol Cell Endocrinol. 1991;79:R21–R24. doi: 10.1016/0303-7207(91)90113-7. [DOI] [PubMed] [Google Scholar]
- 12.Thibonnier M, Coles P, Thibonnier A, et al. The basic and clinical pharmacology of nonpeptide vasopressin receptor antagonists. Annu Rev Pharmacol Toxicol. 2001;41:175–202. doi: 10.1146/annurev.pharmtox.41.1.175. [DOI] [PubMed] [Google Scholar]
- 13.Ventura MA, Rene P, de Keyzer Y, et al. Gene and cDNA cloning and characterization of the mouse V3/V1b pituitary vasopressin receptor. J Mol Endocrinol. 1999;22:251–260. doi: 10.1677/jme.0.0220251. [DOI] [PubMed] [Google Scholar]
- 14.Johnston CA, Siderovski DP. Receptor-mediated activation of heterotrimeric G-proteins: current structural insights. Mol Pharmacol. 2007;72:219–230. doi: 10.1124/mol.107.034348. [DOI] [PubMed] [Google Scholar]
- 15.Thibonnier M. Genetics of vasopressin receptors. Curr Hypertens Rep. 2004;6:21–26. doi: 10.1007/s11906-004-0006-8. [DOI] [PubMed] [Google Scholar]
- 16.Digby GJ, Lober RM, Sethi PR, et al. Some G protein heterotrimers physically dissociate in living cells. Proc Natl Acad Sci USA. 2006;103:17789–17794. doi: 10.1073/pnas.0607116103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamenetsky M, Middelhaufe S, Bank EM, et al. Molecular details of cAMP generation in mammalian cells: a tale of two systems. J Mol Biol. 2006;362:623–639. doi: 10.1016/j.jmb.2006.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deen PM, Verdijk MA, Knoers NV, et al. Requirement of human renal water channel aquaporin-2 for vasopressin-dependent concentration of urine. Science. 1994;264:92–95. doi: 10.1126/science.8140421. [DOI] [PubMed] [Google Scholar]
- 19.Nielsen S, Frøkiaer J, Marples D, et al. Aquaporins in the kidney: from molecules to medicine. Physiol Rev. 2002;82:205–244. doi: 10.1152/physrev.00024.2001. [DOI] [PubMed] [Google Scholar]
- 20.Sabolic I, Katsura T, Verbavatz JM, et al. The AQP2 water channel: effect of vasopressin treatment, microtubule disruption, and distribution in neonatal rats. J Membr Biol. 1995;143:165–175. doi: 10.1007/BF00233445. [DOI] [PubMed] [Google Scholar]
- 21.Sasaki S, Kuwahara M, Yamashita Y, et al. Structure and function of AQP2. Nephrol Dial Transplant. 2000;15(Suppl 6):21–22. doi: 10.1093/ndt/15.suppl_6.21. [DOI] [PubMed] [Google Scholar]
- 22.Bouley R, Sun TX, Chenard M, et al. Functional role of the NPxxY motif in internalization of the type 2 vasopressin receptor in LLC-PK1 cells. Am J Physiol Cell Physiol. 2003;285:C750–C762. doi: 10.1152/ajpcell.00477.2002. [DOI] [PubMed] [Google Scholar]
- 23.Yi X, Bouley R, Lin HY, et al. Alix (AIP1) is a vasopressin receptor (V2R)-interacting protein that increases lysosomal degradation of the V2R. Am J Physiol Renal Physiol. 2007;292:F1303–F1313. doi: 10.1152/ajprenal.00441.2005. [DOI] [PubMed] [Google Scholar]
- 24.Bouley R, Lin HY, Raychowdhury MK, et al. Downregulation of the vasopressin type 2 receptor after vasopressin-induced internalization: involvement of a lysosomal degradation pathway. Am J Physiol Cell Physiol. 2005;288:C1390–C1401. doi: 10.1152/ajpcell.00353.2004. [DOI] [PubMed] [Google Scholar]
- 25.Robben JH, Knoers NV, Deen PM. Regulation of the vasopressin V2 receptor by vasopressin in polarized renal collecting duct cells. Mol Biol Cell. 2004;15:5693–5699. doi: 10.1091/mbc.E04-04-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Besseghir K, Trimble ME, Stoner L. Action of ADH on isolated medullary thick ascending limb of the Brattleboro rat. Am J Physiol. 1986;251:F271–F277. doi: 10.1152/ajprenal.1986.251.2.F271. [DOI] [PubMed] [Google Scholar]
- 27.Firsov D, Mandon B, Morel A, et al. Molecular analysis of vasopressin receptors in the rat nephron. Evidence for alternative splicing of the V2 receptor. Pflugers Arch. 1994;429:79–89. doi: 10.1007/BF02584033. [DOI] [PubMed] [Google Scholar]
- 28.Nonoguchi H, Owada A, Kobayashi N, et al. Immunohistochemical localization of V2 vasopressin receptor along the nephron and functional role of luminal V2 receptor in terminal inner medullary collecting ducts. J Clin Invest. 1995;96:1768–1778. doi: 10.1172/JCI118222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park F, Mattson DL, Skelton MM, et al. Localization of the vasopressin V1a and V2 receptors within the renal cortical and medullary circulation. Am J Physiol. 1997;273:R243–R251. doi: 10.1152/ajpregu.1997.273.1.R243. [DOI] [PubMed] [Google Scholar]
- 30.Schlatter E, Greger R. cAMP increases the basolateral Cl-conductance in the isolated perfused medullary thick ascending limb of Henle's loop of the mouse. Pflugers Arch. 1985;405:367–376. doi: 10.1007/BF00595690. [DOI] [PubMed] [Google Scholar]
- 31.Terada Y, Tomita K, Nonoguchi H, et al. Different localization and regulation of two types of vasopressin receptor messenger RNA in microdissected rat nephron segments using reverse transcription polymerase chain reaction. J Clin Invest. 1993;92:2339–2345. doi: 10.1172/JCI116838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edwards RM, Jackson BA, Dousa TP. ADH-sensitive cAMP system in papillary collecting duct: effect of osmolality and PGE2. Am J Physiol. 1981;240:F311–F318. doi: 10.1152/ajprenal.1981.240.4.F311. [DOI] [PubMed] [Google Scholar]
- 33.Kersting U, Dantzler DW, Oberleithner H, et al. Evidence for an acid pH in rat renal inner medulla: paired measurements with liquid ion-exchange microelectrodes on collecting ducts and vasa recta. Pflugers Arch. 1994;426:354–356. doi: 10.1007/BF00374794. [DOI] [PubMed] [Google Scholar]
- 34.Good DW, Knepper MA. Ammonia transport in the mammalian kidney. Am J Physiol. 1985;248:F459–F471. doi: 10.1152/ajprenal.1985.248.4.F459. [DOI] [PubMed] [Google Scholar]
- 35.Hamm LL. In: Renal acidification in Kidney. Brenner BM, Levine SA, editors. vol 1. Saunders; Philadelphia: 2004. pp. 497–534. [Google Scholar]
- 36.Cohen DM. Signalling and gene regulation by urea and NaCl in the renal medulla. Clin Exp Pharmacol Physiol. 1999;26:69–73. doi: 10.1046/j.1440-1681.1999.02991.x. [DOI] [PubMed] [Google Scholar]
- 37.Jamison RL, Robertson CR. Editorial review. Recent formulations of the urinary concentrating mechanism: a status report. Kidney Int. 1979;16:537–545. doi: 10.1038/ki.1979.163. [DOI] [PubMed] [Google Scholar]
- 38.Knepper MA, Gamba G. In: Urine Concentration and Dilution in Kidney. Brenner BM, Levine SA, editors. vol 1. Saunders; Philadelphia: 2004. pp. 599–636. [Google Scholar]
- 39.Skorecki KL, Conte JM, Ausiello DA. Effects of hypertonicity on cAMP production in cultured renal epithelial cells (LLC-PK1) Miner Electrolyte Metab. 1987;13:165–172. [PubMed] [Google Scholar]
- 40.Innamorati G, Le Gouill C, Balamotis M, et al. The long and the short cycle. Alternative intracellular routes for trafficking of G-protein-coupled receptors. J Biol Chem. 2001;276:13096–13103. doi: 10.1074/jbc.M009780200. [DOI] [PubMed] [Google Scholar]
- 41.Mori T, Cowley AW. Angiotensin II-NAD(P)H oxidase-stimulated superoxide modifies tubulovascular nitric oxide cross-talk in renal outer medulla. Hypertension. 2003;42:588–593. doi: 10.1161/01.HYP.0000091821.39824.09. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Z, Rhinehart K, Kwon W, et al. ANG II signaling in vasa recta pericytes by PKC and reactive oxygen species. Am J Physiol Heart Circ Physiol. 2004;287:H773–H781. doi: 10.1152/ajpheart.01135.2003. [DOI] [PubMed] [Google Scholar]
- 43.Silbernagl S, Volker K, Dantzler WH. Compartmentation of amino acids in the rat kidney. Am J Physiol. 1996;270:F154–F163. doi: 10.1152/ajprenal.1996.270.1.F154. [DOI] [PubMed] [Google Scholar]
- 44.Bagnasco S, Balaban R, Fales HM, et al. Predominant osmotically active organic solutes in rat and rabbit renal medullas. J Biol Chem. 1986;261:5872–5877. [PubMed] [Google Scholar]
- 45.Bagnasco SM, Uchida S, Balaban RS, et al. Induction of aldose reductase and sorbitol in renal inner medullary cells by elevated extracellular NaCl. Proc Natl Acad Sci USA. 1987;84:1718–1720. doi: 10.1073/pnas.84.6.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamauchi A, Sugiura T, Kitamura H, et al. Effects of partial nephrectomy on the expression of osmolyte transporters. Kidney Int. 1997;51:1847–1854. doi: 10.1038/ki.1997.252. [DOI] [PubMed] [Google Scholar]
- 47.Jans DA, Hemmings BA. cAMP-dependent protein kinase activation affects vasopressin V2-receptor number and internalization in LLC-PK1 renal epithelial cells. FEBS Lett. 1991;281:267–271. doi: 10.1016/0014-5793(91)80408-u. [DOI] [PubMed] [Google Scholar]
- 48.Demaurex N. pH Homeostasis of cellular organelles. News Physiol Sci. 2002;17:1–5. doi: 10.1152/physiologyonline.2002.17.1.1. [DOI] [PubMed] [Google Scholar]
- 49.Murphy RF, Powers S, Cantor CR. Endosome pH measured in single cells by dual fluorescence flow cytometry: rapid acidification of insulin to pH 6. J Cell Biol. 1984;98:1757–1762. doi: 10.1083/jcb.98.5.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Edwards BR, LaRochelle FT. Antidiuretic effect of endogenous oxytocin in dehydrated Brattleboro homozygous rats. Am J Physiol. 1984;247:F453–F465. doi: 10.1152/ajprenal.1984.247.3.F453. [DOI] [PubMed] [Google Scholar]
- 51.Lencer WI, Brown D, Ausiello DA, et al. Endocytosis of water channels in rat kidney: cell specificity and correlation with in vivo antidiuresis. Am J Physiol. 1990;259:C920–C932. doi: 10.1152/ajpcell.1990.259.6.C920. [DOI] [PubMed] [Google Scholar]
- 52.Li C, Wang W, Summer SN, et al. Molecular mechanisms of antidiuretic effect of oxytocin. J Am Soc Nephrol. 2008;19:225–232. doi: 10.1681/ASN.2007010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Conrad KP, Gellai M, North WG, et al. Influence of oxytocin on renal hemodynamics and electrolyte and water excretion. Am J Physiol. 1986;251:F290–F296. doi: 10.1152/ajprenal.1986.251.2.F290. [DOI] [PubMed] [Google Scholar]
- 54.Potter RR. Water retention due to oxytocin. Obstet Gynecol. 1964;23:699–702. [PubMed] [Google Scholar]
- 55.Schrier RW. Vasopressin and aquaporin 2 in clinical disorders of water homeostasis. Semin Nephrol. 2008;28:289–296. doi: 10.1016/j.semnephrol.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burgess WJ, Balment RJ, Beck JS. Effects of luminal vasopressin on intracellular calcium in microperfused rat medullary thick ascending limb. Ren Physiol Biochem. 1994;17:1–9. doi: 10.1159/000173782. [DOI] [PubMed] [Google Scholar]
- 57.Ikeda M, Yoshitomi K, Imai M, et al. Cell Ca2+ response to luminal vasopressin in cortical collecting tubule principal cells. Kidney Int. 1994;45:811–816. doi: 10.1038/ki.1994.107. [DOI] [PubMed] [Google Scholar]
- 58.Musabayane CT, Forsling ML, Balment RJ. Arginine vasopressin increases renal sodium excretion in the anesthetized rat through V1 receptors. Ren Fail. 1997;19:23–32. doi: 10.3109/08860229709026257. [DOI] [PubMed] [Google Scholar]
- 59.Maric C, Casley D, Harris P, et al. Angiotensin II binding to renomedullary interstitial cells is regulated by osmolality. J Am Soc Nephrol. 2001;12:450–455. doi: 10.1681/ASN.V123450. [DOI] [PubMed] [Google Scholar]
- 60.Klingler C, Ancellin N, Barrault MB, et al. Potentiation of receptor-mediated cAMP production: role in the cross-talk between vasopressin V1a and V2 receptor transduction pathways. Biochem J. 1998;330(Part 2):1023–1028. doi: 10.1042/bj3301023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Laycock JF, Hanoune J. From vasopressin receptor to water channel: intracellular traffic, constraint and by-pass. J Endocrinol. 1998;159:361–372. doi: 10.1677/joe.0.1590361. [DOI] [PubMed] [Google Scholar]
- 62.Phillips PA, Grant SL, Davidson AF, et al. Epidermal growth factor antagonizes vasopressin in vivo and in vitro. Kidney Int. 1994;45:1028–1036. doi: 10.1038/ki.1994.139. [DOI] [PubMed] [Google Scholar]
- 63.Roy C, Ausiello DA. Regulation of vasopressin binding to intact cells. Ann NY Acad Sci. 1981;372:92–105. doi: 10.1111/j.1749-6632.1981.tb15463.x. [DOI] [PubMed] [Google Scholar]
- 64.Roy C, Hall D, Karish M, et al. Relationship of (8-lysine) vasopressin receptor transition to receptor functional properties in a pig kidney cell line (LLC-PK1) J Biol Chem. 1981;256:3423–3427. [PubMed] [Google Scholar]
- 65.Baudouin-Legros M, Badou A, Paulais M, et al. Hypertonic NaCl enhances adenosine release and hormonal cAMP production in mouse thick ascending limb. Am J Physiol. 1995;269:F103–F109. doi: 10.1152/ajprenal.1995.269.1.F103. [DOI] [PubMed] [Google Scholar]
- 66.DeRubertis FR, Craven PA. Effects of osmolality and oxygen availability on soluble cyclic AMP-dependent protein kinase activity of rat renal inner medulla. J Clin Invest. 1978;62:1210–1221. doi: 10.1172/JCI109241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Riccardi D, Lee WS, Lee K, et al. Localization of the extracellular Ca(2+)-sensing receptor and PTH/PTHrP receptor in rat kidney. Am J Physiol. 1996;271:F951–F956. doi: 10.1152/ajprenal.1996.271.4.F951. [DOI] [PubMed] [Google Scholar]
- 68.Torikai S, Wang MS, Klein KL, et al. Adenylate cyclase and cell cyclic AMP of rat cortical thick ascending limb of Henle. Kidney Int. 1981;20:649–654. doi: 10.1038/ki.1981.189. [DOI] [PubMed] [Google Scholar]
- 69.Baudouin-Legros M, Asdram L, Tondelier D, et al. Extracellular urea concentration modulates cAMP production in the mouse MTAL. Kidney Int. 1996;50:26–33. doi: 10.1038/ki.1996.282. [DOI] [PubMed] [Google Scholar]
- 70.Dousa TP. Effect of renal medullary solutes on vasopressin-sensitive adenyl cyclase. Am J Physiol. 1972;222:657–662. doi: 10.1152/ajplegacy.1972.222.3.657. [DOI] [PubMed] [Google Scholar]
- 71.Santos BC, Chevaile A, Hebert MJ, et al. A combination of NaCl and urea enhances survival of IMCD cells to hyperosmolality. Am J Physiol. 1998;274:F1167–F1173. doi: 10.1152/ajprenal.1998.274.6.F1167. [DOI] [PubMed] [Google Scholar]
- 72.Zhang Z, Tian W, Cohen DM. Urea protects from the proapoptotic effect of NaCl in renal medullary cells. Am J Physiol Renal Physiol. 2000;279:F345–F352. doi: 10.1152/ajprenal.2000.279.2.F345. [DOI] [PubMed] [Google Scholar]
- 73.Lee YJ, Song IK, Jang KJ, et al. Increased AQP2 targeting in primary cultured IMCD cells in response to angiotensin II through AT1 receptor. Am J Physiol Renal Physiol. 2007;292:F340–F350. doi: 10.1152/ajprenal.00090.2006. [DOI] [PubMed] [Google Scholar]
- 74.Wong NL, Tsui JK. Angiotensin II upregulates the expression of vasopressin V2 mRNA in the inner medullary collecting duct of the rat. Metabolism. 2003;52:290–295. doi: 10.1053/meta.2003.50047. [DOI] [PubMed] [Google Scholar]
- 75.Hasler U, Jeon US, Kim JA, et al. Tonicity-responsive enhancer binding protein is an essential regulator of aquaporin-2 expression in renal collecting duct principal cells. J Am Soc Nephrol. 2006;17:1521–1531. doi: 10.1681/ASN.2005121317. [DOI] [PubMed] [Google Scholar]
- 76.Hasler U, Nielsen S, Feraille E, et al. Posttranscriptional control of aquaporin-2 abundance by vasopressin in renal collecting duct principal cells. Am J Physiol Renal Physiol. 2006;290:F177–F187. doi: 10.1152/ajprenal.00056.2005. [DOI] [PubMed] [Google Scholar]
- 77.Kasono K, Saito T, Saito T, et al. Hypertonicity regulates the aquaporin-2 promoter independently of arginine vasopressin. Nephrol Dial Transplant. 2005;20:509–515. doi: 10.1093/ndt/gfh677. [DOI] [PubMed] [Google Scholar]
- 78.Shimizu N, Dean T, Tsang JC, et al. Novel parathyroid hormone (PTH) antagonists that bind to the juxtamembrane portion of the PTH/PTH-related protein receptor. J Biol Chem. 2005;280:1797–1807. doi: 10.1074/jbc.M408270200. [DOI] [PubMed] [Google Scholar]