Figure 3.
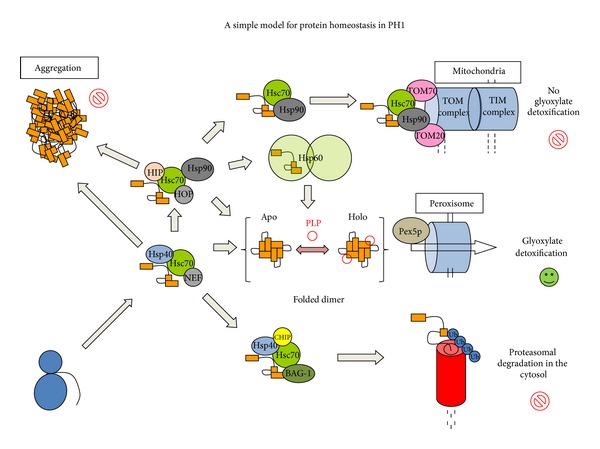
A simple scheme representing potentially important checkpoints in the folding and misfolding of human AGT. After ribosomal synthesis, the AGT monomer is maintained in a partially folded and soluble state upon interaction with Hsp40 chaperones allowing its engagement to the Hsp70 machinery. Correct folding may proceed through the transfer of the partially folded polypeptide to the Hsp90 and Hsp60 machineries leading to the folded holo-AGT dimer and peroxisomal import through the Pex5p import machinery. However, PH1 causing mutants to show enhanced interactions with Hsp70, Hsp60, and Hsp90 chaperone systems which may (i) delay correct folding eventually causing AGT aggregation; (ii) allow engaging the proteasomal degradation machinery mediated by CHIP and BAG-1 proteins; (iii) allow Hsp70/Hsp90 mediated presentation to the mitochondrial import machinery via TOM20 or TOM70 receptors. For further details and references, see the main text.
