Figure 4.
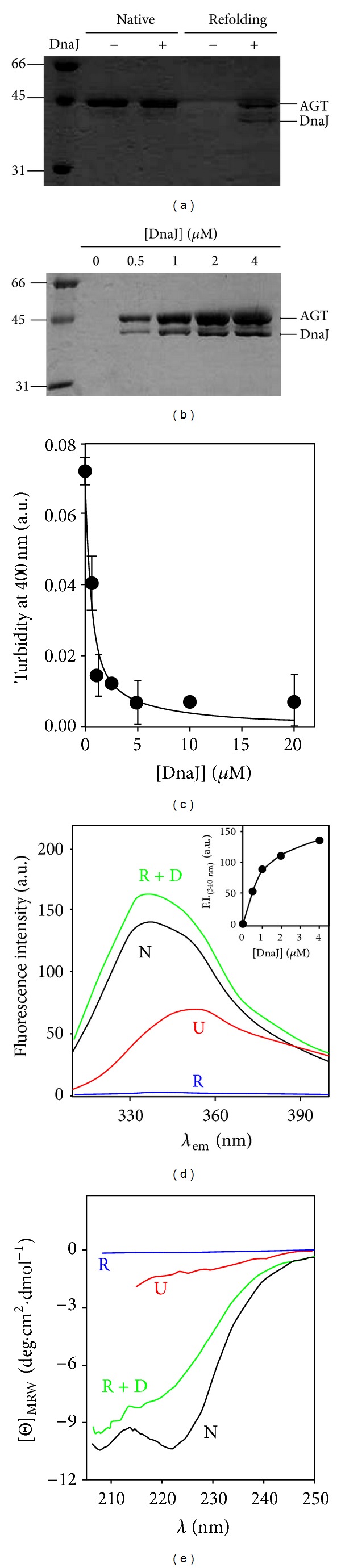
DnaJ prevents aggregation of a partially folded state of AGT. (a) IMAC-based copurification assay of his-tagged apo-AGTwt with DnaJ (1 μM each), under native (no urea) and refolding (20-fold dilution from a 16 h incubated sample with 8 M urea) conditions; (b) DnaJ protein concentration dependence of its interaction with apo-AGTwt under refolding conditions (the same conditions as in (a)). (c) DnaJ protein concentration dependence of the maximal turbidity at 400 nm in the apo-AGTwt refolding (data are from three independent experiments); (d) and (e) solubility and conformational assays of Apo-AGTwt based on its intrinsic Trp-emission fluorescence ((d); exc.295 nm) or Far-UV CD (e) under different conditions: native (0 M urea; N), unfolded (8 M urea; U), and apo-AGTwt refolded in the absence (R) or presence of 4 μM DnaJ (R + D). After urea-dilution, samples were incubated at 25°C for 30 min, centrifuged at 15000 rpm for 30 min, and the spectroscopic analyses were performed in the supernatants. The contribution from DnaJ to fluorescence is negligible due to the absence of Trp residues, while its contribution to Far-UV-CD spectra was subtracted from R + D. Inset: DnaJ protein concentration dependence of the Trp-fluorescence on the soluble fraction. A fitting to a hyperbolic function is shown, providing half-maximal fluorescence recovery at 1.0 ± 0.1 μM DnaJ. All the experiments were performed at 25°C in Na-Hepes 20 mM NaCl 200 mM pH 7.4 2 mM DTT using 1 μM AGT (in protein subunit). DnaJ was purified according to [4].
