Abstract
Numerous species of soil bacteria which flourish in the rhizosphere of plants or around plant tissues stimulate plant growth and reduce nematode population by antagonistic behavior. These bacteria are collectively known as PGPR (plant growth promoting rhizobacteria). The effects of six isolates of PGPR Pseudomonas putida, Pseudomonas fluorescens, Serratia marcescens, Bacillus amyloliquefaciens, Bacillus subtilis and Bacillus cereus, were studied on tomato plant growth and root knot nematode reproduction after 45 days from nematode infection. The highest number of shoot dry weight/g (43.00 g) was detected in the plant treated with S. marcescens; then P. putida (34.33 g), B. amyloliquefaciens (31.66 g), P. fluorescens (30.0 g), B. subtilis (29.0 g), B. cereus (27.0 g) and nematode alone (untreated) 20 g/plant. While the highest number of plant height was observed when plant was treated with S. marcescens, P. fluorescens, P. putida, B. amyloliquefaciens and P. putida 52.66, 50.66, 48 and 48 cm respectively. No significant differences were seen between previous treatments but only had significant differences compared with untreated plant. The highest number of fruit/plant was observed when plants were treated with S. marcescens (10.66), then B. amyloliquefaciens (8.66), P. putida (8), P. fluorescens (8) and B. cereus (7.66). No significant differences between the last 4 treatments, but all had significant differences compared with untreated plants. The highest weight of plant yield (g) was observed with S. marcescens (319.6 g/plant) and the lowest weight of plant yield was observed in plants treated with nematode alone (untreated). On the other hand, the lowest numbers of J2/10 g of soil (78), galls/root, (24.33) galls/root, egg masses/root (12.66) and egg/egg masses were observed in the plants treated with S. marcescens.
Keywords: PGPR, Meloidogyne, Biological control, Rhizobacteria, Pseudomonas
1. Introduction
Root knot nematodes are sedentary obligate endoparasitic nematodes that cause major economic damage to crops around the world (Williamson and Hussey, 1996). Plant parasitic nematodes cause global losses to crop plants with an estimated loss of $ 125 billion per year in the tropics (Chitwood, 2003). Four major species, namely Meloidogyne incognita, Meloidogyne javanica, Meloidogyne hapla and Meloidogyne arenaria have been reported to infect tomatoes, but M. incognita has been found dominant and a major limiting factor in the tomato crop production in major production regions (Maqbool et al., 1988). Second stage juveniles (J2) penetrate the roots and migrate to the vascular cylinder, induce severe root galling and ravage the utilization efficiency of water and nutrients and greatly affect photosynthetic products (McClure, 1977). Consequently the nematode infection of plants leads to foliage symptoms including stunted growth, wilting, and poor fruit yield. Several control strategies, such as host plant resistance, rotation with non-hosts, destruction of residual crop roots, and use of nematicides, have been reported to effectively control root-knot nematodes (Whitehead, 1998). Biological control using microbial antagonists is one potential alternative to chemical nematicides. Among the biological control agents that have been assessed are egg-parasitic fungi, nematode-trapping fungi, bacteria, and polyphagous predatory nematodes (Gray, 1988; Kerry, 1988; Kerry and Hidalgo-Diaz, 2004; Kiewnick and Sikora, 2005; Abdelmoneim, 2006). The challenge of producing fresh fruits and vegetables is increasing for both yield and quality to satisfy consumers avoiding deleterious effects on the environment (Mader et al., 2002). Many marketable biofertilizers are mainly based on plant growth-promoting rhizobacteria (PGPR) that exert beneficial effects on plant development often related to the increment of nutrient availability to host plant (Vessey, 2003). PGPR seem to promote growth through suppression of plant disease-causing organisms (Zehnder et al., 2001; Ji et al., 2006; Veerubommu and Kanoujia, 2011), competition for space, nutrients and ecological niches, production of antimicrobial substances, or through production of phytohormones and peptides acting as bio stimulants without negative effects on the user, consumer or the environment (Glick et al., 1998; Johnsson et al., 1998; Jimenez-Delgadillo, 2004). PGPR have shown positive effects on tomato fruit quality attributes, particularly on size and texture (Hortencia et al., 2007), although on some other parameters such as germination rate, tolerance to drought, weight of shoots and roots, yield, and plant growth under salt stress (Van Loon et al., 1998; Kokalis-Burelle and Dickson, 2003; Kloepper et al., 2004; Yildirim et al., 2006; Kavino et al., 2010; Piromyou et al., 2011). The objective of this study was to determine the effect of tomato root inoculation with six isolates of PGPR on tomato plant performance and root knot nematode M. incognita reproduction under greenhouse conditions.
2. Materials and methods
2.1. Mass culturing of plant growth promoting rhizobacteria (PGPR)
The isolates of PGPR were supplied by microbiology Lab of the Faculty of Agriculture, Suez Canal University, Ismailia, Egypt. PGPR included Pseudomonas putida, Pseudomonas fluorescens, Serratia marcescens, Bacillus amyloliquefaciens, Bacillus subtilis and Bacillus cereus. They were multiplied on nutrient broth. For making the stock solution, their culture was mixed in 100 ml of 5% sugar solution to have the concentration of 2.5 × 106 CFU/ml of each PGPR.
2.2. Nematode inoculums
M. incognita was reared on a tomato plant (Lycopersicon esculentum Mill cv. Rutgers) in the greenhouse (day and night temperature between 28 °C and 20 °C respectively) using a single egg mass from an identified female nematode to make a stock pure culture. Eggs were extracted with 0.5% sodium hypochlorite solution (Hussey and Barker, 1973) from the pure culture when needed as well as the second stage juveniles (J2) were allowed to hatch in a modified Baermann funnel (Pitcher and Flegg, 1968) for 2–3 days and collected on an autoclaved 45 μm sieve.
Three tomato seedlings cv. Rutgers susceptible to M. incognita, were planted in pots (25 cm in diameter), one week later they were thinned to one seedling/pot. Fourteen treatments were replicated three times as following: (1) Plants were inoculated by nematode (M. incognita) alone as 450 J2/plant. (2) Plants were treated with each of the six bacterial isolates by soaking the seedling in the suspension containing approximately 2.5 × 106 cells/ml for 3 min, before planting and inoculated by J2 of M. incognita (450 J2/plant). (3) Plants were treated with the six bacterial isolates by soaking the seedling in the suspension containing approximately 2.5 × 106 cells/ml for 3 min and planting in soil free from nematode infection. (4) Plants were left free from any nematode or bacterial addition to serve as a check. The plants were allowed to grow for 45 days and then harvested to determine the plant growth parameters including shoot dry weight, plant height, number of fruits/plant and weight of yield/plant, as well as the number of J2 in soil, galls per root system and egg-masses/root system were counted.
2.3. Data analysis
Data were analyzed using analysis of variance (ANOVA) by using SAS statistical software (SAS Institute, Cary, NC, USA, 1998). The significance of differences within treatments was separated by Least Significant Difference test at 5%.
3. Results
Strains of PGPR varied in response to control root knot nematode. Data illustrated graphically in Figs. 1 and 2 show the effect of six isolates of PGPR (P. putida, P. fluorescens, S. marcescens, B. amyloliquefaciens, B. subtilis and B. cereus) on the tomato plant growth (shoot dry weight g/plant, plant height/cm, number of fruits/plants and weight of yield/plant g) and nematode reproduction (J2/10 g of soil, galls/root, egg mass/root and egg/egg mass). The highest number of shoot dry weight/g (43.00 g) was detected in the plant treated with S. marcescens; then P. putida (34.33 g), B. amyloliquefaciens (31.66 g), P. fluorescens (30.0 g), B. subtilis (29.0 g), B. cereus (27.0 g) and nematode alone (untreated) 20 g/plant. While the highest number of plant height was observed when plant was treated with S. marcescens, P. fluorescens, P. putida, B. amyloliquefaciens and P. putida 52.66, 50.66, 48 and 48 respectively. No significant differences were seen between previous treatments but only had significant differences compared with untreated plant. The highest number of fruit/plant was observed when plants were treated with S. marcescens (10.66), then B. amyloliquefaciens (8.66), P. putida (8), P. fluorescens (8) and B. cereus (7.66). No significant differences between the last 4 treatments, but all had significant differences compared with untreated plant. The highest weight of plant yield (g) was observed with S. marcescens (319.6 g/plant) and the lowest weight of plant yield was observed in plants treated with nematode alone (untreated). On the other hand, the lowest numbers of J2/10 g of soil (78), galls/root (24.33), egg masses/root (12.66) and egg/egg masses (280.66) were observed in the plants treated with S. marcescens.
Figure 1.
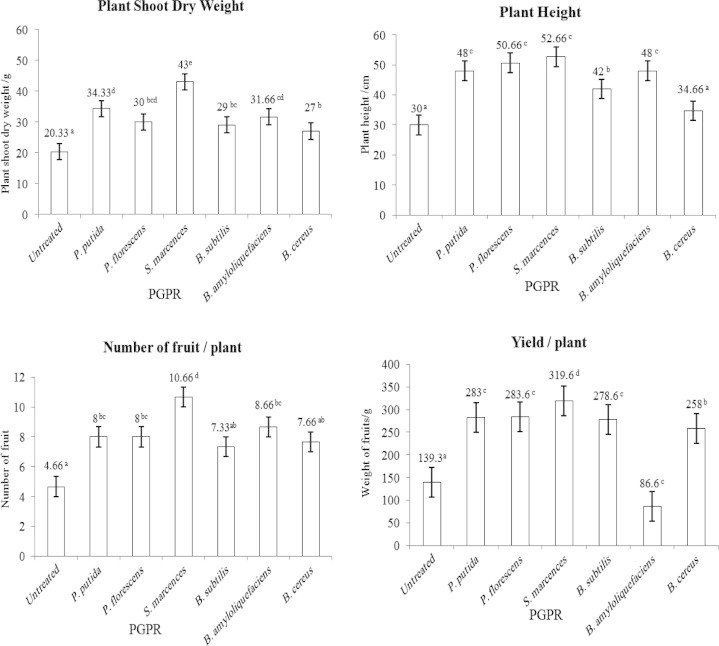
Influence of six isolates of plant growth promoting rhizobacteria (PGPR) on tomato plant performance.
Figure 2.
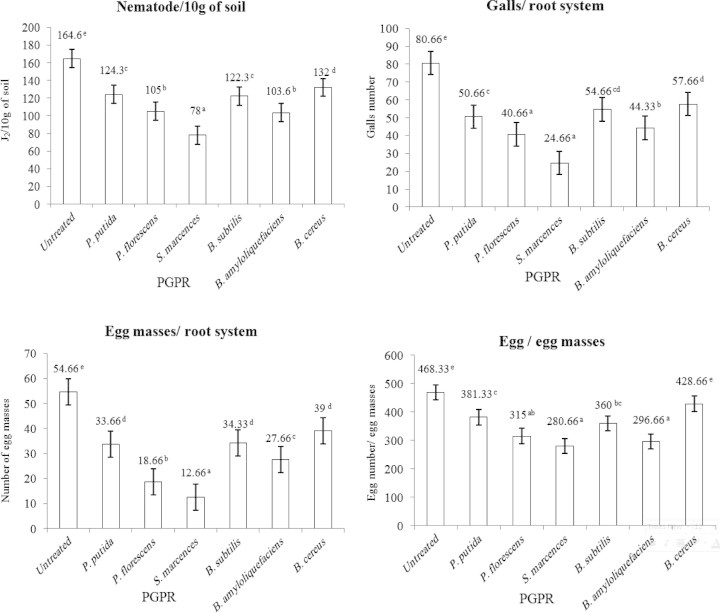
Influence of six isolates of plant growth promoting rhizobacteria (PGPR) on root knot nematode reproduction.
4. Discussion
The use of plant growth promoting rhizobacteria (PGPR) promotes plant growth and development through a variety of mechanisms. The exact mechanism by which PGPR stimulate plant growth is not clearly known, although several mechanisms such as production of phytohormones, suppression of deleterious organisms, activation of phosphate solubilization and promotion of the mineral nutrient uptake are usually believed to be involved in plant growth promotion. The results showed that damage of root knot nematode was reduced by using six strains of PGPR (Martinez-Ochoa, 2000; Zehnder et al., 2001; Lucy et al., 2004; Kloepper and Ryu, 2006). The plant growth promoting rhizobacteria significantly reduced galling and egg masses on the roots by root-knot nematodes in tomato crops and resulted in increased yield (Siddiqui et al., 2001; Kokalis-Burelle and Dickson, 2003). PGPR have been reported to improve plant growth either through direct stimulation by the synthesis of phytohormones (Xie et al., 1996) or by decreasing the effect of pathogens (Weller, 1988; Weller et al., 2002). Some rhizobacteria (Bacillus spp.) have been found to produce lipopeptides, surfactins, bacillomycin D, and fengycins, which are secondary metabolites mainly with inhabitant pathogen activity (Chen et al., 2006). Also some species of Pseudomonas bacteria were recorded as highly aggressive colonizers of the rhizosphere of various crop plants and has a broad spectrum antagonistic activity against plant pathogens like nematodes (Parveen et al., 1998; Raaijmakers and Weller, 2001; Li et al., 2002; Weller et al., 2002). In addition to some species of Pseudomonas Bacillus are reported to induce systemic resistance in plants against invading pathogens and antagonists to root-knot nematodes of Meloidogyne spp. (Zhou and Paulitz, 1994; Wei et al., 1996; De Meyer et al., 1999; Siddiqui et al., 2001; Kloepper et al., 2004; Kloepper and Ryu, 2006). The reduction of galls and number of egg masses by PGPR, as found in our study, agrees with Kloepper et al. (1991), Kloepper et al. (1999), Siddiqui et al. (2001), Ali et al. (2002), Li et al. (2002), Siddiqui and Shaukat (2002) and Kokalis-Burelle and Dickson (2003).
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdelmoneim, T.S., 2006. Using some microorganisms or their products for control of plant parasitic nematodes. PhD. Thesis, Faculty of Agriculture, Suez Canal University, Ismailia, Egypt, pp. 1–111.
- Ali N.I., Siddiqui I.A., Shaukat S.S., Zaki M.J. Nematicidal activity of some strains of Pseudomonas spp. Soil Biol. Biochem. 2002;34:1051–1058. [Google Scholar]
- Chen X.H., Vater J., Piel J., Franke P., Scholz R., Schneider K., Koumoutsi A., Hitzeroth G., Grammel N., Strittmatter A.W., Gottschalk G., Sussmuth R.D., Borriss R. Structural and functional characterization of three polyketide synthase gene clusters in Bacillus amyloliquefaciens FZB 42. J. Bacteriol. 2006;188:4024–4036. doi: 10.1128/JB.00052-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitwood D.J. Research on plant parasitic nematode biology conduct by the United States department of agriculture, agriculture research service. Pest Manag. Sci. 2003;59:748–753. doi: 10.1002/ps.684. [DOI] [PubMed] [Google Scholar]
- De Meyer G., Capiau K., Audenaert K., Buchala A., Metraux J.P., Hofte M. Nanogram amounts of salicylic acid produced by the rhizobacterium Pseudomonas aeruginosa 7NSK2 activate the systemic acquired resistance pathway in bean. Mol. Plant Microbe Interact. 1999;2:450–458. doi: 10.1094/MPMI.1999.12.5.450. [DOI] [PubMed] [Google Scholar]
- Glick B.R., Penrose D.M., Li J.P. A model for the lowering of plant ethylene concentrations by plant growth-promoting bacteria. J. Theor. Biol. 1998;190:63–68. doi: 10.1006/jtbi.1997.0532. [DOI] [PubMed] [Google Scholar]
- Gray N.F. Ecology of nematophagous fungi: effect of the soil nutrients N, P and K, and seven major metals on distribution. Plant Soil. 1988;108:286–290. [Google Scholar]
- Hortencia G.M., Olalde V., Violante P. Alteration of tomato fruit quality by root inoculation with plant growth-promoting rhizobacteria (PGPR): Bacillus subtilis BEB-13bs. Sci. Hortic. 2007;113:103–106. [Google Scholar]
- Hussey R., Barker K. A comparison of methods of collecting inocula of Meloidogyne spp., including a new technique. Plant Dis. Rep. 1973;57:1025–1028. [Google Scholar]
- Ji P., Campbell H., Kloepper J., Jones J., Suslow T., Wilson M. Integrated biological control of bacterial speck and spot of tomato under field conditions using foliar biological control agents and plant growth-promoting rhizobacteria. Biol. Control. 2006;36:358–367. [Google Scholar]
- Jimenez-Delgadillo, M.R., 2004. Peptidos Secretados por Bacillus subtilis que Codifican la Arquitectura de la Raiz de Arabidopsis thaliana. PhD. Dissertation, CINVESTAV, Unidad Irapuato, MX.
- Johnsson L., Hökeberg M., Gerhardson B. Performance of the Pseudomonas chlororaphis biocontrol agent MA 342 against seed-borne diseases in field experiments. Eur. J. Plant Pathol. 1998;104:701–711. [Google Scholar]
- Kavino M., Harish S., Kumar N., Kumar S., Samiyappan R. Effect of chitinolytic PGPR on growth, yield and physiological attributes of banana (Musa spp.) under field conditions. Appl. Soil Ecol. 2010;45:71–77. [Google Scholar]
- Kerry B.R. Fungal parasites of cyst nematodes. Agric. Ecosyst. Environ. 1988;24:293–305. [Google Scholar]
- Kerry B.R., Hidalgo-Diaz L. Application of Pochonia chlamydosporia in the integrated control of root-knot nematodes on organically grown vegetable crops in Cuba. IOBC WPRS Bull. 2004;27:23–26. [Google Scholar]
- Kiewnick S., Sikora R. Biological control of the root-knot nematode Meloidogyne incognita by Paecilomyces lilacinus strain 251. Biol. Control. 2005;38:179–187. [Google Scholar]
- Kloepper J.W., Ryu C.M. Bacterial endophytes as elicitors of induced systemic resistance. In: Schulz B., Boyle C., Siebern T., editors. Microbial Root Endophytes. Springer-Verlag; Heildelberg: 2006. pp. 33–51. [Google Scholar]
- Kloepper J.W., Rodrguez-kbana R., Mcinroy J.A., Collins D.J. Analysis of populations and physiological characterization of microorganisms in rhizospheres of plants with antagonistic properties to phytopathogenic nematodes. Plant Soil. 1991;136:95–102. [Google Scholar]
- Kloepper J.W., Rodriguez-Kabana R., Kenney D.S., Reddy M.S., Martinez-Ochoa N., Kokalis-Burelle N., Arthur K. Development of an integrated biological approach to develop transplants suppressive to various plant diseases. Phytopathology. 1999;89:S40. [Google Scholar]
- Kloepper J.W., Ryu C.M., Zhang S. Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology. 2004;94:1259–1266. doi: 10.1094/PHYTO.2004.94.11.1259. [DOI] [PubMed] [Google Scholar]
- Kokalis-Burelle, N., Dickson, D.W., 2003. Effects of soil fumigants and bioyield tm on root knot nematode incidence and yield of tomato. In: Proc. Int. Res. Conf. Methyl Bromide Alternatives and Emissions Reductions, vol. 50, pp. 1-50.
- Li W., Roberts D.P., Dery P.D., Meyer S.L.F., Lohrke S., Lumsden R.D., Hebbar K.P. Broad spectrum anti-biotic activity and disease suppression by the potential biocontrol agent Burkholderia ambifaria BC-F. Crop Prot. 2002;21:129–135. [Google Scholar]
- Lucy M., Reed E., Glick B.R. Applications of free living plant growth promoting rhizobacteria. Antonie Van Leeuwenhoek. 2004;86:1–25. doi: 10.1023/B:ANTO.0000024903.10757.6e. [DOI] [PubMed] [Google Scholar]
- Mader P., Fliessbach A., Dubois D., Gunst L., Fried P., Niggli U. Soil fertility and biodiversity in organic farming. Science. 2002;296:1694–1697. doi: 10.1126/science.1071148. [DOI] [PubMed] [Google Scholar]
- Maqbool M.A., Hashmi S., Ghaffar A. Problem of root knot nematode in Pakistan and strategy for their control. In: Maqbool M.A., Golden A.M., Ghaffar A., Krusberg L.R., editors. Advances in Plant Nematology. National Nematological Research Centre, University of Karachi; Karachi, Pakistan: 1988. pp. 229–240. [Google Scholar]
- Martinez-Ochoa, N., 2000. Biological control of the root-knot nematode with rhizobacteria and organic amendments. PhD. Dissertation, Auburn University, Alabama, p. 120.
- McClure M.A. Meloidogyne incognita: a metabolic sink. J. Nematol. 1977;9:88–90. [PMC free article] [PubMed] [Google Scholar]
- Parveen S., Ehteshamul-Haque S., Ghaffar A. Efficacy of Pseudomonas aeruginosa and Paecilomyces lilacinus in the control of root rot-root knot disease complex of some vegetables. Nematologia Mediterr. 1998;26:209–212. [Google Scholar]
- Piromyou P., Buranabanyat B., Tantasawat P., Tittabutr P., Boonkered N., Teaumroong N. Effect of plant growth promoting rhizobacteria (PGPR) inoculation on microbial community structure in rhizosphere of forage corn cultivated in Thailand. Eur. J. Soil Biol. 2011;47:44–54. [Google Scholar]
- Pitcher R.S., Flegg J.J. An improved final separation sieve for the extraction of plant parasitic nematodes from soil debris. Nematologica. 1968;14:123–127. [Google Scholar]
- Raaijmakers J.M., Weller D.M. Exploiting genotypic diversity of 2, 4-diacetylphloroglucinol-producing Pseudomonas spp. characterization of superior root-colonizing P. fluorescens strain Q8r1-96. Appl. Environ. Microbiol. 2001;67:2545–2554. doi: 10.1128/AEM.67.6.2545-2554.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute . 6th edition. SAS Institute Inc.; North Carolina, Cary, Inc.: 1988. SAS/STAT User’s Guide. Release 6.03 Edition. pp. 1028. [Google Scholar]
- Siddiqui I.A., Shaukat S.S. Resistance against damping-off fungus Rhizoctonia solani systematically induced by the plant-growth-promoting rhizobacteria Pseudomonas aeruginosa (1E–6S (+)) and P. fluorescens (CHAO) J. Phytopathol. 2002;150:500–506. [Google Scholar]
- Siddiqui I.A., Ehetshamul-Haque S., Shaukat S.S. Use of rhizobacteria in the control of root rot-root knot disease complex of mungbean. J. Phytopathol. 2001;149:337–346. [Google Scholar]
- Van Loon L.C., Bakker P.A.H.M., Pieterse C.M.J. Systemic resistance induced by rhizosphere bacteria. Annu. Rev. Phytopathol. 1998;36:453–483. doi: 10.1146/annurev.phyto.36.1.453. [DOI] [PubMed] [Google Scholar]
- Veerubommu S., Kanoujia N. Biological management of vascular wilt of tomato caused by Fusarium oxysporum f.sp. lycospersici by plant growth-promoting rhizobacterial mixture. Biol. Control. 2011;57:85–93. [Google Scholar]
- Vessey J.K. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil. 2003;255:571–586. [Google Scholar]
- Wei G., Kloepper J.W., Tuzun S. Induced systemic resistance to encounter decreases and increased plant growth promoting bacteria under field conditions. Phytopathology. 1996;86:221–224. [Google Scholar]
- Weller D.M. Biological control of soilborne plant pathogens in the rhizosphere with bacteria. Annu. Rev. Phytopathol. 1988;26:379–407. [Google Scholar]
- Weller D.M., Raaijmakers J.M., Mcspadden B.B., Thomashow L.S. Microbial populations responsible for specific soil suppressiveness to plant pathogens. Annu. Rev. Phytopathol. 2002;40:309–348. doi: 10.1146/annurev.phyto.40.030402.110010. [DOI] [PubMed] [Google Scholar]
- Whitehead A.G. CAB International; Wallingford, UK: 1998. Plant Nematode Control. pp. 384. [Google Scholar]
- Williamson V.M., Hussey R.S. Nematode pathogenesis and resistance in plants. Plant Cell. 1996;8:1735–1745. doi: 10.1105/tpc.8.10.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H., Pasternak J.J., Glick B.R. Isolation and characterization of mutants of the plant growth-promoting rhizobacterium Pseudomonas putida GR12-2 that over produce indoleacetic acid. Curr. Microbiol. 1996;32:67–71. [Google Scholar]
- Yildirim E., Taylor A.G., Spittler T.D. Ameliorative effects of biological treatments on growth of squash plants under salt stress. Sci. Hortic. 2006;111:1–6. [Google Scholar]
- Zehnder G.W., Murphy J.F., Sikora E.J., Kloepper J.W. Application of rhizobacteria for induced resistance. Eur. J. Plant Pathol. 2001;107:39–50. [Google Scholar]
- Zhou T., Paulitz T.C. Induced resistance in the biocontrol of Pythium aphanidermatum by Pseudomonas spp., on cucumber. J. Phytopathol. 1994;142:51–63. [Google Scholar]



