Abstract
This study aims to investigate the effects of inoculation using Terfeziaboudieri Chatin ascospores (ectomycorrhizal fungus) on growth, root colonization and nutrient status of Helianthemumsessiliflorum Desf. seedlings grown in pots on two-soil types (gypseous and sandy loam). Mycorrhizal seedlings had significantly increased their height and leaf number compared to non-mycorrhizal ones. Regardless of mycorrhizal inoculation treatments, the plants growing on gypseous soil showed higher growth as compared to sandy loam one. It appears that inoculation with T. boudieri changed root morphology, increasing branching of first-order lateral roots of H. sessiliflorum seedlings. The highest root mycorrhizal colonization was recorded in inoculated seedlings on sandy loam soil (89%) when compared to gypseous one (52%). N, P and K concentrations in mycorrhizal seedlings were significantly improved by fungal inoculation. It can be concluded that inoculation of H. sessiliflorum with T. boudieri increased growth attributes and improved plant nutritional status.
Keywords: Helianthemum sessiliflorum, Terfezia boudieri, Growth, Mineral nutrition, Mycorrhizae, Tunisia
1. Introduction
Many plant species naturally establish mycorrhizal associations to resist abiotic and biotic stresses such as drought, salinity, cold and nutrient deficiency (Bethlenfalvay and Linderman, 1992; Querejeta et al., 2003). According to Querejeta et al. (2003), the mycorrhizal hyphae persisted in soils with water potential of −20 MPa after prolonged drought. These authors concluded that direct water translocation, maintaining mycorrhizal activity, could potentially improve the nutrient status of deep-rooted plants when the upper soil layer is dry.
Among mycorrhizal fungi, desert truffles are nutritious hypogeous mushrooms exhibiting unusual biological features and are ecto-, ectendo- or endo-mycorrhiza depending on the external conditions (Kagan-Zur and Roth-Bejerano, 2008). The truffles host plant species vary with the ecological and climatic conditions characterizing their habitats. Several Terfezia and Tirmania species form mycorrhizas with both perennial (Roth-Bejerano et al., 1990) and annual (Awameh and Alsheikh, 1978; Morte et al., 2000; Kovac’s et al., 2003) Cistaceae species roots especially Helianthemum genus (Awameh, 1981; Dexheimer et al., 1985; Roth-Bejerano et al., 1990; Morte et al., 1994; Dickie et al., 2004; Slama et al., 2010). Only few studies have investigated the effects of ectomycorrhizal (ECM) fungi inoculation on the growth and mycorrhizal infection of some Helianthemum species such as Helianthemum salicifolium (L.) Mill. and Helianthemum ledifolium (L.) Mill. (Awameh, 1981).
Helianthemum sessiliflorum is an endemic perennial dwarf shrub distributed in many sandy regions in southern Tunisia. According to Le Floc’h (1983) and Slama et al. (2006, 2010), this plant is usually related to desert truffles and can establish a symbiotic association with them. In this study, we investigate the effect of this symbiotic relation on growth attributes, mycorrhizal root colonization and nutrient status of H. sessiliflorum seedlings.
2. Materials and methods
2.1. Materials
H. sessiliflorum seeds were collected in March 2003 from Ben Guardene region (33°17′N, 10°46′E; southeast Tunisia) characterized by irregular rainfall events and harsh dry summer. Annual precipitation is around 186 mm and annual mean temperature is 19.4 °C with a minimum temperature 3.9 °C in January and 35.9 °C maximum in August (Maraghni et al., 2010). Seeds were cleaned and stored in the seed bank of the Laboratoire d’Ecologie Pastorale at the Institut des Régions Arides (Médenine, Tunisia) during six months with 30% relative humidity and 20 °C temperature. Terfezia boudieri fruiting bodies were collected in April 2004 from the same region. Ascocarps were sun-dried during 2 months and stored at 20 °C. Two soil types were used in this study. The first is sandy loam sampled from Ben Guardene (organic matter = 1.17%, K = 27.27 ppm, total phosphorus = 575 ppm, EC = 353 μs cm−1, pH = 7.1). The second is gypseous sampled from an experimental field at the Institut des Régions Arides (33°30’N, 10°38’E; southeast Tunisia) (organic matter = 3.45%, K = 12.8 ppm, total phosphorus = 212 ppm, EC = 469 μs cm−1, pH = 7.7). Both soils were 2 mm-sieved and sterilized before H. sessiliflorum cultivation. The experiment design was two levels of root inoculant treatment (inoculated and non-inoculated) × two soil types (sandy loam and gypseous) × five harvests (1, 2, 3, 4 and 5 months) × 10 replicates. Plastic pots (1-L volume, 7.5 cm base and 12 cm top diameters) were sterilized and filled with the considered soil types. These pots were perforated at the bottom and contained a disinfected layer of gravel to ensure drainage of water.
2.2. Plant cultivation and mycorrhizal inoculation at time of sowing
For each pot, 0.5 g of H. sessiliflorum seeds were surface scarified and sown. Mix of fungal fragments and deionised water was prepared and added to pot according to Chevalier et al. (1973) and Chevalier and Grente (1979). Inoculated and non-inoculated seedlings were grown in a growth chamber with 23 °C ± 1 °C temperature, 50% day and 75% night relative humidity and 16 h light/8 h dark regime. Capillar irrigation was applied twice a week with tap water during the experimental period (154 days).
2.3. Determination of growth attributes
Plant height and leaf number of inoculated and non-inoculated seedlings were measured 74 days after sowing. Ten days interval measurements were than made until the end of the experiment.
2.4. Determination of mycorrhization percentage
Ten seedlings, from each treatment and for both soil types, were monthly harvested since the first month after sowing until the end of the experiment to assess their root colonization rate. Sampled roots were washed free of soil and stored in 70% ethanol. Roots were cleared with 10% KOH and stained with 0.5% acidic Fuchine according to the method of Phillips and Hayman (1970). Stained roots were observed using Leica DMLS microscope (Leica Microsystems, Bannockburn, IL) and the frequency of mycorrhizal colonization in the root system was determined as F (%) = (n/N) × 100, where N is the total number of observed root fragments and n is the number of mycorrhized root fragments (Trouvelot et al., 1986).
2.5. Determination of inorganic solutes in seedlings
At the end of the experiment, K, N and P were determined in 10 plants per treatment after nitric perchloric digestion of the finely grounded dry matter. K was assayed by atomic absorption spectrophotometry (Schimazu AA 6800, Kyoto, Japan), while N and P were colorimetrically assayed, by Kjeldahl and molybdovanadate methods, respectively, using a spectrophotometer (Jenway 6400 London, UK).
2.6. Statistical analysis
Data were analysed using SPSS for Windows, version 11.5. A two-way analysis of variance (ANOVA) was carried out to test the main factors (treatment and soil type) effects and their interaction on plant growth attributes and mycorrhizal root colonization.
3. Results
3.1. Growth attributes
The ANOVA for plant height and leaf number showed significant differences between treatment, soil type and their interaction (Table 1). Compared to non-inoculated controls, inoculated plants have the highest emergence percentages and growth. Both growth attributes (height and leaf number) of H. sessiliflorum evolved in a similar way and were significantly improved by mycorrhizal inoculation (Fig. 1a and b). Regardless of mycorrhizal inoculation treatments, the plants growing under gypseous soil showed higher growth as compared to the sandy loam one. At the end of the experiment, the height and leaf number of inoculated seedlings were about 2.8- and 3-fold higher than non-inoculated controls in gypseous soil, while these parameters were 3.9- and 7.4-fold higher than non-inoculated controls in sandy loam soil, respectively. Regardless of mycorrhizal inoculation treatment, the plants growing on gypseous soil showed higher growth attributes compared to those growing on sandy loam soil. At the end of the experiment, the height and leaf number of inoculated seedlings were about 2.8- and 3-fold and 3.9- and 7.4-fold higher than non-inoculated controls in gypseous and sandy loam soils, respectively.
Table 1.
Results of two-way ANOVA of traits related to plant height, leaf number and root mycorrhizal colonization of Helianthemumsessiliflorum seedlings by treatment, soil type and their interaction.
| Source of variation | Dependent variables |
||
|---|---|---|---|
| Plant height | Leaf number | Mycorrhizal colonization | |
| Treatment (Tr) | 636.65∗∗∗ | 73.48∗∗∗ | 296.20∗∗∗ |
| Soil type (S) | 266.95∗∗∗ | 152.75∗∗∗ | 35.61∗∗∗ |
| Tr × S | 10.59∗∗ | 5.80∗ | 35.61∗∗∗ |
Numbers are F-values significant at ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Figure 1.

Changes in leaf number (a) and plant height (b) of inoculated and non-inoculated Helianthemum sessiliflorum seedlings grown on two soil types. Measurements were started 74 days after sowing with 10 day intervals until the end of the experiment (154 days). Data represent mean ± 95% confidence limits (n = 10).
3.2. Mycorrhizal colonization
A two-way ANOVA of root mycorrhizal colonization indicated a significant main effect of salt type, osmotic potential and their interaction (Table 1). Mycorrhization percentage showed a significant increase in inoculated seedlings, whereas no infection was observed in non-inoculated controls (Fig. 2). The highest mycorrhizal colonization was recorded on sandy loam soil when compared to gypseous one. At the end of experimental period, the mycorrhization percentage reached 89% and 52% in sandy loam and gypseous soils, respectively.
Figure 2.
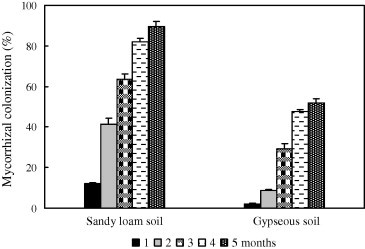
Mycorrhizal colonization percentage of Helianthemum sessiliflorum seedlings, inoculated with Terfezia boudieri ascospores, grown on two soil types. Measurements were started one month after sowing with 30 day intervals until the end of the experiment (5 months). Data represent mean ± 95% confidence limits (n = 10).
3.3. Nutrient status
N, P and K concentrations in inoculated H. sessiliflorum seedlings were significantly improved by mycorrhizal inoculation; however, soil type did not significantly influence their nutrient status, except N (Table 2). Furthermore, the interaction treatment × soil type had no effect on inorganic solute concentrations. At the end of the experimental period, N, P and K concentrations were about 1.3-, 2.4- and 4.6-fold and 1.2-, 1.8- and 5.4-fold higher than non-inoculated controls in sandy loam and gypseous soils, respectively (Table 2).
Table 2.
Changes in N (%), K and P (mg g−1 dry weight) concentrations in inoculated (I) and non-inoculated (NI) Helianthemumsessiliflorum seedlings grown on two soil types.
| Soil type | Treatment | K (mg g−1) | P (mg g−1) | N (%) |
|---|---|---|---|---|
| Sandy loam | NI | 2.74 | 0.99 | 0.51 |
| I | 12.63 | 2.35 | 0.64 | |
| Gypseous | NI | 2.07 | 1.07 | 0.34 |
| I | 11.28 | 1.97 | 0.42 | |
| ANOVA | d.f. | F | F | F |
| Treatment (Tr) | 1 | 42.17⁎⁎⁎ | 9.59⁎ | 164.09⁎⁎⁎ |
| Soil type (S) | 1 | 0.47ns | 0.17ns | 46.87⁎⁎⁎ |
| Tr × S | 1 | 0.05ns | 0.40ns | 3.12ns |
For significance the numbers are F-value according to two-way ANOVA showing the effects of treatment, soil type and their interaction.
p < 0.05.
p < 0.001.
Not significant.
4. Discussion
As shown in the results, H. sessiliflorum seedlings colonized by T. boudieri were characterized by a higher growth than non-inoculated controls. Working on H. salicifolium and H. ledifolium, Awameh (1981) reported that leaves of inoculated plants by the desert truffle T. boudieri were longer, bigger and more numerous than non-inoculated controls. At 64 and 94 days, the same author showed that the height of inoculated plants irrigated with a solution without nutrient was three times greater than that of non-inoculated control seedlings. Roth-Bejerano et al. (1990) have also observed a better growth in inoculated plants of H. sessiliflorum with Terfezia leonis Tul. than non-inoculated controls. Mycorrhized plants result from the association between roots and fungal hyphae, obtained after ascospores germination. The relationship between the two partners was assured by the development of mycorrhizae, implying improvement of plant water and nutritional status by fungal external hyphae. Our data show that the roots of inoculated plants were more branched and greater than those of non-mycorrhizal controls. These observations corroborate those of Awameh (1981) and Morte et al. (2000) showing that the roots of Helianthemum species were more developed in inoculated plants when compared to non-inoculated ones. In the present experiment, 5-month old seedlings of H. sessiliflorum inoculated with T. boudieri in sandy loam soil presented a mycorrhizal colonization (89%) similar to those found by Fortas and Chevalier (1992) on mycorrhizas between Helianthemum guttatum and Terfezia arenaria (91.1%), Terfezia claveryi (92.3%), and Tirmania pinoyi (69.3%) in enriched soil. These authors obtained for the same species, in nutrient-deficient soil, a root mycorrhization rate of 96.7%, 96.8% and 98.6%, respectively.
Almost mycorrhizal fungi develop an external mycelium that connected the roots of plants with the surrounding soil particles and soil solution and maintained water and nutrient uptake to the associate plant through the hyphae structure that promote an enhanced absorption (Boulois et al., 2008). Symbiotic mycorrhizal fungi play an important role in the absorption of soil nutrients and water by most plants. Trappe (1981) indicated that the high degree of nutrient absorption from the soil is due to the activity of specific assimilative enzymes in mycorrhizal plants. Our data show higher contents of nitrogen and phosphorus in inoculated plants than non-inoculated controls. As indicated by Marx (1975), mycorrhizal symbiosis is essential for the growth and health of many herbaceous plants in non-fertile soils. Numerous studies have revealed that symbiotic associations abound in non-fertile soils and constitute an essential factor to unite plants to the mushrooms (Delmas, 1989; Loewe et al., 2000; Hertel et al., 2003) and ensure water uptake (Smith and Read, 1997; Chalot and Brun, 1998; Plassard et al., 2002) and mineral nutrition (Marschner, 1995; Lindahl et al., 1999) by fungal hyphae. In the Mediterranean areas where water constituted a restrictive factor for the plant growth, Domínguez Núñez et al. (2006) signalled that mycorrhizal association promoted seedling growth by the fungal water uptake. It is currently accepted that mycorrhizae have a positive effect on plant nutrient uptake and growth since the accessible soil volume for plants is enlarged by mycelial conduction (Harley and Smith, 1983; Smith and Read, 1997). Colonized by either T. arenaria or T. claveryi, H. guttatum shifts from ectomycorrhizae at high phosphate concentration to endomycorrhizae at low concentration (Fortas and Chevalier, 1992). Moreover, Helianthemum almeriense colonized by T. claveryi, encountered only endomycorrhizae under field conditions, only ectomycorrhizae in vitro, and mostly ectomycorrhizae lacking a mantle in pots under greenhouse conditions (Gutierrez et al., 2003). Cameleyre and Olivier (1993) suggested that mycorrhizae of Tuber melanosporum liberate phosphatases and organic ions, which are able to absorb phosphorus. These authors concluded that in Mediterranean ecosystems where soils are generally deficient in phosphorus, mycorrhizal associations are beneficial to plant nutrition and survival. Abuzinadah et al. (1986) confirmed the importance of microbial biomass as a major source of organic N for mycorrhizal plants. Moreover, Chalot and Brun (1998) proposed that assimilation of organic nitrogen by ECM roots may ameliorate nitrogen nutrition especially in soils characterized by high organic matter content. As shown in results, inoculated plants showed higher K content than controls. Few data related to Tuber spp. inoculation effect on K content were found but no studies on Terfezia effects were achieved. Delmas (1989) and Marschner (1995) reported that mycorrhizae have a beneficial effect in absorption of potassium by host plants.
5. Conclusions
From these results, it is clear that mycorrhizal fungus T. boudieri improved the growth attributes and nutrient status of inoculated seedlings of H. sessiliflorum when compared to non-inoculated controls. The introduction of fungi in desert dry lands is a promising alternative to improve the vegetation cover in these fragile areas to combat desertification and to help in the economical development of rural and local populations.
References
- Abuzinadah R.A., Finlay R.D., Read D.J. The role of proteins in the nitrogen nutrition of ectomycorrhizal plants. II Utilization of protein mycorrhizal plants of Pinus contorta. New Phytol. 1986;103:495–506. [Google Scholar]
- Awameh M.S. The response of Helianthemum salicifolium and H. ledifolium to infection by the desert truffle Terfezia boudieri. Mushroom Sci. 1981;11:843–853. [Google Scholar]
- Awameh M.S., Alsheikh A. Laboratory and field study of four kinds of truffle (Kamah), Terfezia and Tirmania species, for cultivation. Mushroom Sci. 1978;10:507–517. [Google Scholar]
- Bethlenfalvay G.J., Linderman R.G. American Society of Agronomy; Madison: 1992. Mycorrhizae in Sustainable Agriculture. [Google Scholar]
- Boulois H.D., Joner E.J., Leyval C., Jakobsen I., Chen B.D., Roos P., Thiry Y., Rufyikiri G., Delvaux B., Declerck S. Role and influence of mycorrhizal fungi on radiocesium accumulation by plants. J. Environ. Radioact. 2008;99:785–800. doi: 10.1016/j.jenvrad.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Cameleyre I., Olivier J.M. Evidence for intraspecific isozymes variations among French isolates of Tuber melanosporum Vitt. FEMS Microbiol. Lett. 1993;110:159–162. [Google Scholar]
- Chalot M., Brun A. Physiology of organic nitrogen acquisition by ectomycorrhizal fungi and ectomycorrhizas. FEMS Microbiol. Rev. 1998;22:21–44. doi: 10.1111/j.1574-6976.1998.tb00359.x. [DOI] [PubMed] [Google Scholar]
- Chevalier G., Grente J. Application pratique de la symbiose ectomycorhizienne: production à grande échelle de plants mycorhizés par la truffe (Tuber melanosporum Vitt) Mushroom Sci. 1979;10:483–505. [Google Scholar]
- Chevalier G., Grente J., Pollacsek A. Obtention de mycorhizes de différents Tuber par synthèse à partir de spores en conditions gnotoxéniques et à partir de cultures pures de mycélium en condition axéniques et gnotoxéniques. Ann. Phytopathol. 1973;5:107–108. [Google Scholar]
- Delmas J. Flammarion, La Maison Rustique; Paris: 1989. (Les champignons et leur culture. Culture actuelle et potentielle des champignons supérieurs). [Google Scholar]
- Dexheimer J., Gerard J., Leduc J.P., Chevalier G. Etude ultrastructurale comparée des associations symbiotiques mycorhiziennes Helianthemum salicifolium-Terfezia claveryi et Heliathemum salicifolium-Terfezia leptoderma. Can. J. Bot. 1985;63:582–591. [Google Scholar]
- Dickie I.A., Guza R.C., Krazewski S.E., Reich P.B. Shared ectomycorrhizal fungi between a herbaceous perennial (Helianthemum bicknellii) and oak (Quercus) seedlings. New Phytol. 2004;164:375–382. doi: 10.1111/j.1469-8137.2004.01177.x. [DOI] [PubMed] [Google Scholar]
- Domínguez Núñez J.A., Selva Serrano J., Rodríguez Barreal J.A., Saiz de Omeñaca González J.A. The influence of mycorrhization with Tuber melanosporum in the afforestation of a Mediterranean site with Quercus ilex and Quercus faginea. For. Ecol. Manage. 2006;231:226–233. [Google Scholar]
- Fortas Z., Chevalier G. Effet des conditions de culture sur la mycorhization de l’Helianthemum guttatum par trois espèces de terfez des genres Terfezia et Tirmania d’Algérie. Can. J. Bot. 1992;70:2453–2460. [Google Scholar]
- Gutierrez A., Morte A., Honrubia M. Morphological characterization of the mycorrhiza formed by Helianthemum almeriense Pau with Terfezia claveryi Chatin and Picoa lefebvrei (Pat) Maire. Mycorrhiza. 2003;13:299–307. doi: 10.1007/s00572-003-0236-7. [DOI] [PubMed] [Google Scholar]
- Harley J.L., Smith S.E. Academic Press; New York: 1983. Mycorrhizal Symbiosis. p. 483. [Google Scholar]
- Hertel D., Leuschnep C., Hölscher D. Size and structure of fine root systems in old-growth and secondary tropical montane forests (Costa Rica) Biotropica. 2003;35:143–153. [Google Scholar]
- Kagan-Zur V., Roth-Bejerano N. Desert truffles. Fungi. 2008;1:32–37. [Google Scholar]
- Kovac’s G.M., Vágvölgyi C., Oberwinkler F. In vitro interaction of the truffle Terfezia terfezoïdes with Robinia pseudoacacia and Helianthemum ovatum. Folia Microbiol. 2003;48:369–378. doi: 10.1007/BF02931369. [DOI] [PubMed] [Google Scholar]
- Le Floc’h, E., 1983. Contribution à une étude ethnobotanique de la flore Tunisienne. Imprimerie Officielle de la République Tunisienne, Tunis.
- Lindahl B.R., Stenlid J., Olsson S., Finalay R. Translocation of P-32 between interacting mycelia of a wood-decomposing fungus and ectomycorrhizal fungi in microcosm systems. New Phytol. 1999;144:183–193. [Google Scholar]
- Loewe A., Einig W., Shi L., Dizengremel P., Hampp R. Mycorrhiza formation and elevated CO2 both increase the capacity for sucrose synthesis in source leaves of spruce and aspen. New Phytol. 2000;145:565–574. doi: 10.1046/j.1469-8137.2000.00598.x. [DOI] [PubMed] [Google Scholar]
- Maraghni M., Gorai M., Neffati M. Seed germination at different temperatures and water stress levels, and seedling emergence from different depths of Ziziphus lotus. S. Afr. J. Bot. 2010;76:453–459. [Google Scholar]
- Marschner H. second ed. Academic Press; London: 1995. Mineral Nutrition of Plants. p. 889. [Google Scholar]
- Marx D.H. Mycorrhizae and establishment of trees on strip-mined land. Ohio J. Sci. 1975;75:288–297. [Google Scholar]
- Morte M.A., Cano A., Honrubia M., Torres P. ln vitro mycorrhization of micropropagated Helianthemum almeriense plantlets with Terfezia claveryi (desert truffle) Agric. Sci. Finland. 1994;3:309–314. [Google Scholar]
- Morte A., Lovisolo C., Schubert A. Effect of drought stress on growth and water relations of the mycorrhizal association Helianthemum almeriense-Terfzia claveryi. Mycorrhiza. 2000;10:115–119. [Google Scholar]
- Phillips J., Hayman D. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970;55:158–161. [Google Scholar]
- Plassard C., Guérin-Laguette A., Véry A.-A., Casarin V., Thibaud J.-B. Local measurements of nitrate and potassium fluxes along roots of maritime pine. Effects of ectomycorrhyzal symbiosis. Plant Cell Environ. 2002;25:75–84. [Google Scholar]
- Querejeta J.I., Egerton-Warburton L.M., Allen M.F. Direct nocturnal water transfer from oaks to their mycorrhizal symbionts during severe soil drying. Oecologia. 2003;134:55–64. doi: 10.1007/s00442-002-1078-2. [DOI] [PubMed] [Google Scholar]
- Roth-Bejerano N., Livne D., Kagan-Zur V. Helianthemum-Terfezia relations in different growth media. New Phytol. 1990;114:235–238. [Google Scholar]
- Slama A., Fortas Z., Neffati M., Khabar L., Boudabous A. Etude taxinomique de quelques Ascomycota hypogés (Terfeziaceae) de la Tunisie méridionale. Bull. Soc. Mycol. Fr. 2006;122:187–195. [Google Scholar]
- Slama A., Fortas Z., Boudabous A., Neffati M. Cultivation of an edible desert truffle (Terfezia boudieri Chatin) Afr. J. Microbiol. Res. 2010;4:2350–2356. [Google Scholar]
- Smith S.E., Read D.J. second ed. Academic Press; London: 1997. Mycorrhizal Symbiosis. [Google Scholar]
- Trappe J.M. Mycorrhizae and productivity of arid and semiarid rangelands. In: Manassah J.T., Briskey E.J., editors. Advances in Food Producing Systems for Arid and Semiarid Lands. Academic Press; New York: 1981. pp. 581–599. [Google Scholar]
- Trouvelot E., Kough J.-L., Gianinazzi-Pearson V. Mesure du taux de mycorhization VA d’un système radiculaire. Recherche et méthodes d’estimation ayant une signification fonctionnelle. In: Gianinazzi-Pearson V., Gianinazzi S., editors. The Mycorrhizae: Physiology and Genetic. INRA Press; Paris: 1986. pp. 217–221. [Google Scholar]


