Abstract
Background and objectives
This study aimed to assess the dose-dependent effect of antioxidants in protection against cardiovascular changes induced by exposure to cigarette smoke.
Design and setting
This was an experimental study, conducted at King Fahd Medical Research Center, King Abdulaziz University.
Materials and methods
This study was carried out on 57 male albino rats divided into nine groups. Rats of experimental groups were exposed to cigarette smoke from a total of 100 cigarettes per week for four weeks in a specially designed chamber. The antioxidants used (vitamin C, E, and B-carotene) were administrated at low (9, 7.2, and 0.27 mg/day) and high doses (18, 14.4, and 0.54 mg/day), respectively, through gastric feeding tubes. The lipid profile was estimated, and the carotids and heart were removed, weighed, and then processed, and the carotid intima-media thickness was measured. Statistical analysis was performed using the Statistical Package for Social Sciences.
Results
The lipid profile was significantly improved in all groups treated with low or high doses of antioxidants after or during the exposure to cigarette smoke. Improvement was marked in the group treated with a high dose of antioxidants.
The histological changes, as well as the intima-medial thickness of the carotid artery induced by exposure to cigarette smoke, have been improved by treatment with antioxidants (at either low or high doses), either after or during exposure to cigarette smoke. Improvement was marked in the group treated with a low dose of antioxidant. Treatment with antioxidants could not improve degenerated cardiac muscle fibers, while they could reduce the thickness of the branches of the coronary vessels.
Conclusion
These results indicated that antioxidants ameliorated the cigarette smoke contribution to atherosclerosis, but they could not completely reverse the changes induced by cigarette smoke. Simultaneous intake of antioxidants could ameliorate the cigarette-smoke-induced changes apart from those of the heart.
Keywords: Antioxidants, Atherosclerosis, Cigarette smoke, Histological, Biochemical
1. Introduction
Smoking causes an estimated 1.69 million deaths per year worldwide (Ezzati and Lopez, 2003). Extensive evidence has demonstrated that cigarette smoking (Howard et al., 1998) or exposure to secondhand tobacco smoke (Barnoya and Glantz, 2005) increases the risk for cardiovascular disease (CVD). It has also been found that smoking is strongly and positively associated with an increase in the risk for myocardial infarction, sudden cardiac death, and an increase in thrombosis and atherosclerosis (Kannel et al., 1984; Smith and Fischer, 2001). It has been shown that exposure to cigarette smoking (CS) increases myocardial oxygen demand and concurrently reduces coronary blood flow by causing vasoconstriction in the coronary arteries and microvasculature (Bogren et al., 1989; Kiowski et al., 1994).
The mechanism for the increased risk of vascular dysfunction is not well understood, but it is presumed to be due to the absorption of tobacco smoke constituents that affect endothelial cell function, which is a characteristic feature of CVD (Powell and Higman, 1994; Conklin et al., 2009).
The risk of CVD among smokers may vary because of differences in smoking characteristics, such as number of years smoked, number of cigarettes per day, use of filters, inhalation pattern, and type of tobacco, or because of differences in antioxidant vitamin intake or status (Higenbottam et al., 1982; Powell et al., 1997).
Many studies have shown that antioxidant nutrient supplements, especially B-carotene, vitamin C, and vitamin E, are effective in protecting the oxidation of DNA, LDL, and protein against oxidative damage by smoking in vitro (Baker et al., 1999; Panda et al., 1999). Some previous studies also showed that vitamin E has a role in increasing blood flow and arterial vasodilatation in coronary heart diseases (Dutta and Dutta, 2003). It was found that vitamin C is important for the resynthesis of vitamin E from the tocopheroxyl radical; hence it increases the efficacy of vitamin E as an antioxidant (Carr and Frei, 2000). From all of the above, it is clear why this study uses a combination of vitamin C, E, in addition to B-carotene.
The risk of CVD in asymptomatic smokers can be estimated by the intima-media wall thickness (IMT) of the common carotid artery. Moreover, several epidemiological studies show associations between CVD risk factors and carotid IMT (Heiss et al., 1991; Joensuu et al., 1994; Wagenknecht et al., 1997). These include studies reporting positive associations between smoking and IMT (Howard et al., 1994; Howard et al., 1998) and inverse associations between specific antioxidant vitamins and IMT (Bonithon-Kopp et al., 1997; Iribarren et al., 1997).
Reviewing the available literature, few studies have specifically investigated the dose-dependent effect of antioxidants in protection against cardiovascular changes induced by exposure to cigarette smoke. So a primary objective of this study was to assess such effects using both carotid IMT and lipid profile tests, which are known to indicate atherosclerosis. Furthermore, this study aimed to answer the questions: “Could antioxidants reverse the changes induced by exposure to cigarette smoke after its stoppage?” and “Could the simultaneous intake of antioxidants ameliorate these changes?”
2. Materials and methods
This experimental study was carried out on 51 adult male albino (Wister) rats aged two months. Their body weights ranged from 150 to 200 g. The study was carried out at the King Fahd Medical Research Center (KFMRC) and included two experiments. Experiment I aimed to study the effect of the antioxidants dose (low and high dose) administrated orally for four weeks to the animals after stopping exposure to CS. This experiment included four groups, with six rats in each group, which were fed a standard diet for seven weeks:
-
(1)
The negative healthy control group (C0) was left without exposure to CS.
-
(2)
The positive control group (C1) was exposed to CS for three weeks; then exposure was stopped for four weeks.
-
(3)
The first experimental group (E1) was exposed to CS for three weeks and then treated with low dose antioxidants for four weeks after stopping the exposure to CS.
-
(4)
The second experimental group (E2) was exposed to CS for three weeks and then treated with high dose antioxidants for four weeks after stopping the exposure to CS.
Experiment II aimed to study the effect of the antioxidant doses (low and high dose), which were administrated orally for four weeks to the animals during their exposure to CS. This experiment included three groups, with six rats in each group, which were fed a standard diet for four weeks:
-
(1)
The positive control group (C2) was exposed to CS for four weeks.
-
(2)
The third experimental group (E3) was exposed to CS for four weeks, simultaneously with the administration of low doses of antioxidant.
-
(3)
The fourth experimental group (E4) was exposed to CS for four weeks, simultaneously with the administration of high doses of antioxidants.
An additional three control groups, with three rats per group, were compared to the negative control (C0) group to identify the effect of antioxidants used on the structure of the tissues. They included rats (C3 and C4) that were given low doses and high doses of antioxidants, respectively, and rats (C5) that were given corn oil, used to dissolve vitamin E and B-carotenes.
The brand of cigarette used in this study was one of the most commonly used cigarette types in the Saudi community. Rats were exposed to CS from four cigarettes per hour, five times a day (a total of 20 cigarette per day), five days per week (a total of 100 cigarettes per week) for four weeks in a specially designed chamber, following the method described by Ueta et al. (2003), with some modification (Fig. 1A). The antioxidants administrated were vitamins C, E, and B-carotene. They were administrated at low doses (9, 7.2 and 0.27 mg/day, respectively) or at high doses (18, 14.4 and 0.54 mg/day, respectively). These doses of antioxidants were detected according to the method used by Chao et al. (2002) after being adjusted for the rats’ weights, following the method used by Paget and Barnes, (1964).
Figure 1.

(A) Photomicrograph showing the special designed chamber used for exposing the rats to CS according to the method described by Ueta et al. (2003). (B) Photomicrograph of part of a section of control rat carotid artery showing layers of its wall how they were measured using the pro-image analyzer program.
These doses were selected because Chao et al. (2002) found that combined antioxidant vitamin supplements improve the overall antioxidant-protection capacity and reduce the oxidative stress in male hyperlipidemic smokers by increasing antioxidant levels and antioxidative enzyme activities. They also found that higher doses of antioxidant vitamin supplements had no further improved effects on the antioxidative enzyme system and lipid peroxidation.
Vitamin C was dissolved in 1 ml water, while vitamin E and B-carotene were dissolved in 1 ml of corn oil; all of them were administrated separately through gastric feeding tubes.
In experiment I, blood samples were collected from the orbital vein after the rats fasted overnight (12 h) at the end of the third week (after exposure to CS) and at the end of the seventh week (after four weeks of treatment with antioxidants), according to the method used by Waynforth (1988). Regarding experiment II, blood samples were collected at the end of the seventh week only. These blood samples were used for the estimation of cholesterol, triglycerides, LDL, and HDL using the Dimension R clinical chemistry system (Tietz, 1994).
For the histological study, animals were sacrificed by cervical decapitation, and the heart was exposed via thoracic incision. Intracardiac perfusion was administered with normal saline followed by 10% neutral buffered formalin. Carotids were exposed and dissected out; their middle segment (5 mm) was cut, freed from surrounding tissue, and weighed. The heart was removed and weighed, and a circular segment (4 mm thick) just above the apex was obtained. Tissues were refixed in 10% neutral buffered formalin and processed further. Five-micron-thick paraffin sections were obtained and stained with hematoxylin, eosin, and Masson trichrome (Bancroft and Cook, 1994). They were examined and photographed by light microscope with a digital camera (Olympus BX-51). The carotid intima-media thickness was measured using an image Pro-image analyzer system (Fig. 1B).
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS). The data were expressed as mean and standard deviation. The percentages of the studied organs were calculated from total body weight. A Student’s t test was used to compare the two groups, and a paired t test was used to compare serial measurements for the same group. A one-way ANOVA F test was used to compare more than two groups. A least significant difference (LSD) test followed the one-way ANOVA to compare each of the two groups. Significance was considered when p value was less than 0.05.
3. Results
3.1. Effect on carotid artery weight
Regarding experiment I, there was a significant increase in both the carotid artery weight and carotid index (the percent of its weight in relation to the body weight of the animal) of the group exposed to CS for three weeks (C1) compared to the negative control group. The two parameters in the groups treated with either low or high doses of antioxidants, after stopping the exposure to CS, were significantly reduced compared to the positive control (C1) group (Table 1).
Table 1.
Effect of antioxidants on organs weight (mg) and percent of organs weight in relation to the body weight of different studied groups.
| Groups | Carotid artery |
Heart |
||
|---|---|---|---|---|
| Mean ± SD | % Of body weight | Mean ± SD | % Of body weight | |
| Experiment I | ||||
| (C0) Negative control | 0.005 ± 0.000 | 0.002 ± 0.002 | 0.891 ± 0.059 | 0.31 ± 0.021 |
| (C1) Positive control group (Smoking 3 weeks) | 0.007 ± 0.001 | 0.003 ± 0.002 | 0.915 ± 0.067 | 0.33 ± 0.022 |
| (E1) Low doses of antioxidants after 3 weeks exposure to CS | 0.005 ± 0.001 | 0.002 ± 0.003 | 0.966 ± 0.067 | 0.35 ± 0.023 |
| (E2) High doses of antioxidants after 3 weeks exposure to CS | 0.005 ± 0.001 | 0.002 ± 0.001 | 0.914 ± 0.057 | 0.33 ± 0.021 |
| F | 19.84⁎⁎ | 28.47⁎⁎ | 3.32⁎ | 3.76⁎ |
| LSD | 0.001 | 0.000 | 0.097 | 0.028 |
| Experiment II | ||||
| (C0) Negative control | 0.005 ± 0.000 | 0.002 ± 0.001 | 0.891 ± 0.059 | 0.31 ± 0.021 |
| (C2) Positive control group (smoking 4 weeks) | 0.014 ± 0.002 | 0.005 ± 0.002 | 0.946 ± 0.072 | 0.36 ± 0.023 |
| (E3) Low doses of antioxidants during exposure to CS for 7 weeks | 0.008 ± 0.001 | 0.004 ± 0.002 | 0.945 ± 0.073 | 0.36 ± 0.032 |
| (E4) High doses of antioxidants during exposure to CS for 7 weeks | 0.004 ± 0.000 | 0.002 ± 0.001 | 0.787 ± 0.108 | 0.30 ± 0.032 |
| F | 72.67⁎⁎ | 55.86⁎⁎ | 7.89⁎⁎ | 9.82⁎⁎ |
| LSD | 0.002 | 0.001 | 0.112 | 0.029 |
Significant at 0.05.
Significant at 0.01.
For experiment II, these two parameters of the group exposed to CS for four weeks (C2) were significantly increased compared to the negative control group. The two parameters in the group treated with high doses of antioxidants during exposure to CS were significantly lower than those of the positive control (C2) group (Table 1).
3.2. Effect of antioxidants on heart weight
Regarding experiment I, it was noticed that the weight of the heart and the heart index (the percent of its weight in relation to the body weight of the animal) of the group exposed to CS were significantly higher compared to the negative control (C0) group. Heart weight continued to increase even after treatment with a low dose of antioxidants, while a high dose could slightly reduce this increase (Table 1).
In experiment II, there was a significant increase in the heart weight as well as the percentage of its weight in relation to body weight in the group exposed to CS for four weeks compared to the negative control (C0). Administration of high doses of antioxidants succeeded in reducing both parameters, while low doses failed to do that (Table 1).
3.3. Effect on the lipid profile
Regarding experiment I, the cholesterol level was increased in the group exposed to CS for three weeks compared to the negative control (C0) group. Cholesterol level, as well as the percent of its decrease, was significantly decreased in C2, in which the rats were exposed to CS for three weeks, and then the exposure was stopped for the next four weeks without treatment. The percentage of decrease in the cholesterol level was significant in the groups treated with either low or high doses of antioxidants after stopping the exposure to CS. The highest significant decrease was noticed in the group treated with high doses of antioxidant (22.24%) (Table 2). The same changes were noticed in the levels of LDL; the highest percentage of decrease in the LDL level was recorded in the group treated with a high dose of antioxidants (39.11%) (Table 2).
Table 2.
Effect of antioxidants on the lipid profile of the animal of the experiment I in mg/100 ml serum.
| (C0) Negative control | (C1) Positive control (smoking 3 weeks) | (E1) Low doses of antioxidants after 3 weeks exposure to CS | (E2) High doses of antioxidants after 3 weeks exposure to CS | F test, LSD | |
|---|---|---|---|---|---|
| Choles. | |||||
| After 3 weeks | 61.65 ± 2.71 | 73.69 ± 5.32 | 73.69 ± 5.32 | 73.69 ± 5.32 | |
| After 7 weeks | 59.26 ± 4.72 | 66.09 ± 2.84⁎ | 68.47 ± 3.22⁎ | 57.20 ± 4.03⁎⁎ | 17.37⁎⁎, 3.85 |
| % Of change | 3.56– | 9.91– | 6.79– | 22.24– | |
| TG | |||||
| After 3 weeks | 40.86 ± 7.12 | 54.72 ± 14.34 | 54.72 ± 14.34 | 54.72 ± 14.34 | |
| After 7 weeks | 39.53 ± 6.81 | 65.05 ± 10.15 | 47.79 ± 24.10 | 38.21 ± 8.29⁎ | 5.12⁎⁎, 16.45 |
| % Of change | 0.22– | 25.29 | 6.30– | 25.76– | |
| LDL | |||||
| After 3 weeks | 28.93 ± 8.3 | 46.31 ± 6.68 | 46.31 ± 6.68 | 46.31 ± 6.68 | |
| After 7 weeks | 30.92 ± 7.3 | 32.21 ± 2.01⁎⁎ | 38.01 ± 6.19⁎ | 27.35 ± 7.60⁎⁎ | 4.35⁎, 5.91 |
| % Of change | 10.09– | 29.29– | 16.97– | 39.11– | |
| HDL | |||||
| After 3 weeks | 22.42 ± 2.31 | 16.42 ± 3.31 | 16.42 ± 3.31 | 16.42 ± 3.31 | |
| After 7 weeks | 20.61 ± 5.52 | 20.29 ± 0.80⁎⁎ | 20.61 ± 4.46⁎ | 21.20 ± 4.17⁎⁎ | 0.07, 4.40 |
| % Of change | 7.37 | 28.03 | 28.88 | 29.19 | |
Choles.: cholesterol.
TG: triglycerides.
LDL: Low density lipoproteins.
HDL: high density lipoproteins.
p < 0.05.
p < .01.
For experiment II, the level of cholesterol was increased in the group exposed to smoking for four weeks compared to the negative control (C0). The cholesterol level was significantly decreased in the groups treated with either low or high doses of antioxidants during the exposure to CS below the cholesterol level of the negative control group. The same changes were noticed in the level of LDL (Table 3).
Table 3.
Effect of antioxidants on the lipid profile of the animal of the second experiment in mg/100 ml serum.
| Cholesterol | TG | LDL | HDL | |
|---|---|---|---|---|
| (C0) Negative control | 61.65 ± 2.75 | 40.86 ± 7.18 | 28.93 ± 8.30 | 22.42 ± 2.31 |
| (C2) Positive control group (Smoking 4 weeks) | 63.00 ± 4.68 | 57.67 ± 17.40 | 32.86 ± 6.35 | 18.68 ± 1.08 |
| (E3) Low doses of antioxidants during exposure to CS for 7 weeks | 52.88 ± 4.90 | 35.26 ± 4.96 | 25.77 ± 2.62 | 20.10 ± 3.15 |
| (E4) High doses of antioxidants during exposure to CS for 7 weeks | 55.59 ± 7.72 | 34.66 ± 7.73 | 27.51 ± 4.75 | 21.45 ± 2.64 |
| F test | 4.79⁎ | 6.03⁎⁎ | 1.51 | 3.22⁎ |
| LSD | 6.59 | 14.74 | 7.70 | 2.73 |
Choles.: cholesterol.
TG: triglycerides.
LDL: Low density lipoproteins.
HDL: high density lipoproteins.
p < 0.05.
p < .01.
Regarding experiment I, there was an insignificant decrease in the levels of triglycerides in the groups treated with low doses of antioxidants after stopping exposure to CS; the high doses of antioxidants resulted in a significant decrease, and the percentage of decrease was 25.29% (Table 2). Experiment II revealed that there was a significant decrease in the level of triglycerides in the groups treated with low or high doses of antioxidants during their exposure to CS compared to the negative control; the percent of decrease were 33.96% and 35.1%, respectively (Table 3).
The level of HDL decreased by 14.2% in the group exposed to CS for three weeks compared to the negative control. By the end of experiment I, the level of HDL increased even without treatment. There was a significant increase in HDL level in the groups treated with low or high doses of antioxidants after stopping exposure to CS compared to the negative control; the percentages of increase were 28.88% and 29.19%, respectively (Table 2). For experiment II, the level of HDL decreased by 16.7% in the group exposed to CS for four weeks compared to the negative control. HDL level increased in the groups treated with low and high doses of antioxidants during exposure to CS compared to the negative control (Table 3).
3.4. Effect on the histological structure of carotid artery
The carotid arteries of rats from the negative control group (C0) that received a standard diet had relatively wide lumens and thin walls. The arteries had intact structures (Fig. 2A). The structure of the carotid arteries of the control rats that received low doses (C3) or high doses (C4) of antioxidants, or corn oil (C5) appear similar to the negative control arteries, with no histological changes (Fig. 2B–D).
Figure 2.

Photomicrograph of carotid artery of negative control rat showing that its wall consists of very thin intima lined by endothelial cells (arrow), the intima merges with the underlying media which is formed mainly of wavy elastic fibers (bi-head arrow) intermingled with smooth muscle fibers identified by their large oval nuclei (white arrow). Adventitia (Av) is formed of slightly dense connective tissue. Insert shows the carotid artery with wide lumen and the thin wall. (B–D) Carotid arteries of control groups (C3), (C4) and (C5) that received low dose of antioxidants, high dose of antioxidants and corn oil, respectively. All of them appear normal with no changes from that negative control (H&E).
Exposure to CS for three weeks resulted in the loss of some endothelial cells, damage of elastic fibers, and vacuolation of cytoplasm of some smooth muscles of the intima-media layer. An increase in the number of mast cells was observed in the adventitia (Fig. 3A). Stopping the exposure of CS and then starting treatment with a low dose of the antioxidants (E1 group) resulted in marked improvement of the structure of the carotid artery, especially the endothelial lining, compared to the untreated group (C1) (Fig. 3B). On the other hand, treatment with a high dose of antioxidants (E2) resulted in relative improvement in the carotid artery structure compared to the untreated group (C1), as endothelial cells were lost in some areas and damaged in others (Fig. 3C).
Figure 3.
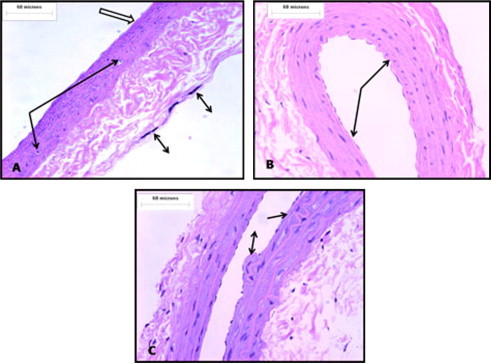
(A) Exposure to CS for 3 week (C1), results in loss of endothelial cells and vacuolation of smooth muscles (thin arrow) of media, increased adventitial thickness and presence of many mast cells (bi-head arrow). (B) Treatment with low dose antioxidants after stopping exposure to CS (E1) results in marked improvement of endothelial lining (arrow) compared to the untreated group (C1). (C) Treatment with high dose antioxidants after stopping exposure to CS (E2) results in relative improvement of the endothelial cells as some cells are lost (arrow) and others are damaged (bi-head arrow) (H&E).
Exposure to CS for four weeks (C2) resulted in the loss of the endothelial cell lining, marked damage of the intimal-medial layer, and marked increase in the adventitial layer thickness, which showed many mast cells and lipid-containing cells (Fig. 4A). Treatment with antioxidants at low (E3) and high (E4) doses simultaneously with exposure to CS resulted in improvement of these changes, apart from the loss of some endothelial cells that was evident in the group treated with high doses of antioxidants (Fig. 4B and C).
Figure 4.

Photomicrographs showing that (A) Exposure to CS for 4 week (C2), results in damage of intimal/medial layer and loss of endothelial cells lining (white arrow). Marked increase thickness of adventitial layer, presence of many mast cells (bi-head arrow) and lipid containing cells (arrow) can be observed. (B and C) Exposure to CS for 4 week during treatment with antioxidants at low (E3) and high (E4) doses results in improvement of the changes induced by CS apart from loss of some endothelial cells which is marked in group treated with high dose antioxidants (H&E).
There was a significant increase in the IMT of the carotid arteries in the group exposed to CS for three and four weeks compared to the negative control (C0) (Table 4). Regarding the effect of antioxidants on the IMT, there was a significant decrease in the IMT of the group treated with both low and high doses of antioxidants after stopping exposure to CS when compared to the positive control (C1) (Table 4). Also, there was a significant decrease in the IMT of the group treated with both low and high doses of antioxidants during exposure to CS when compared to the positive control (C2). It was noticed that the decrease in the IMT in the group that received antioxidants during exposure was more than that in the group that received antioxidants after stopping exposure to CS. It was also noticed that there was a significant increase in IMT in the group that received high doses of antioxidants alone (without exposure to CS) when compared to the negative control (Table 4).
Table 4.
Effect of antioxidants on IMT of the carotid artery (in μm).
| Groups | Range | Mean ± SD |
|---|---|---|
| The 1st experiment | ||
| (C0) Negative control | 51.71–26.84 | 37.61 ± 6.31 |
| (C1) Positive control (smoking 3 weeks) | 87.28–35.51 | 59.33 ± 19.13⁎⁎⁎ |
| (E1) Low doses of antioxidants after 3 weeks exposure to CS | 59.22–25.46 | 41.64 ± 8.94⁎⁎ |
| (E2) High doses of antioxidants after 3 weeks exposure to CS | 67.79–24.87 | 50.18 ± 9.66⁎⁎ |
| The 2nd experiment | ||
| (C0) Negative control | 51.71–26.84 | 37.61 ± 6.31 |
| (C2) Positive control (smoking 4 weeks) | 154.70–23.92 | 62.79 ± 40.63⁎⁎⁎ |
| (E3) Low doses of antioxidants during exposure to CS for 7 weeks | 30.92–23.49 | 27.02 ± 2.19⁎⁎⁎ |
| (E4) High doses of antioxidants during exposure to CS for 7 weeks | 50.18–24.57 | 36.7 ± 9.35⁎⁎ |
| (C3) Control low doses of antioxidant alone | 30.92–23.49 | 27.02 ± 2.19⁎⁎ |
| (C4) Control high doses of antioxidant alone | 86.92–80.51 | 84.38 ± 2.35⁎,⁎⁎⁎ |
| (C5) Control corn oil alone | 66.57–45.52 | 53.11 ± 6.44, NS |
All groups are compared to negative control
C4 was compared to negative control and C3.
p < 0.05.
p < .0.01.
p < 0.001.
3.5. Effect on the histological structure of heart and its vessels
The hearts of the negative control rats showed striated, branching and anastomosing cardiac muscle fibers running in different directions and possessed central oval nuclei. Branches of coronary vessels appeared in between fibers and were surrounded by small amounts of loose connective tissue (Fig. 5A). Hearts of the control groups (C3, C4, and C5) that received low and high doses of antioxidants and corn oil (respectively) appeared normal, with no changes compared to the negative control (C0) group (Fig. 5B–D).
Figure 5.

Photomicrograph of (A) heart of negative control (C0) rat showing cardiac muscle fibers (arrow) with central oval nuclei surrounding one of the branches of coronary vessels (a). The latter is surrounded by small amount of collagen fibers (∗) as seen in the insert stained with Massion trichrome. (B–D) Part of the heart of the control groups (C3, C4 and C5) that received low, high dose of antioxidants and corn oil, respectively. All of them appear intact like the negative control (C0) (H&E).
The hearts of rats (C1) exposed to CS for three weeks showed that the cardiac muscle fibers were widely separated by connective tissue, and some of them appeared deeply stained with dark nuclei (degenerated). This connective tissue showed some inflammatory and mast cells. Branches of the coronary vessels appeared thickened and surrounded by an increased amount of connective tissue (Fig. 6).
Figure 6.

Photomicrograph of heart of positive control (C1) rat showing that cardiac muscle fibers are widely separated by connective tissue (arrow) and some of them appear deeply stained with dark nuclei (head arrow). This connective tissue showed some inflammatory and mast cells (white arrow). One of the branches of the coronary vessels (a) is seen, it appears thickened and surrounded by increased amount of collagen fibers (∗) as seen in the insert stained with Massion trichrome (H&E).
The hearts from the rats that received low doses of antioxidants after stopping the exposure to CS (E1) showed some fibers that appeared with deeply stained cytoplasm and enlarged nuclei (showing hyaline degeneration) (Fig. 7A). The hearts from the rats that received high doses of antioxidants (E2) showed some atrophied cardiac muscle fibers, with some mast cells appearing in the connective tissue in between. Branches of the coronaries appeared of normal thickness, while the thickness of the surrounding connective tissue increased slightly (Fig. 7B). The same changes were observed in the second experiment in rats’ hearts that were treated with antioxidants during the exposure to CS (Figs. 8 and 9).
Figure 7.

(A) Heart of a rat from (E1) group received low dose of antioxidants after stopping the exposure to CS. Most of cardiac muscle fibers appear normal apart from some which possess deeply stained cytoplasm (arrow) and enlarged nuclei. (B) Heart of rat from (E2) received high dose of antioxidants after stopping the exposure to CS. Some cardiac muscle fibers appear atrophied (arrow) and mast cells (white arrow) appear in the connective tissue in between. A branch of the coronaries (a) appears normal with connective tissue around slightly increased (∗) (H&E).
Figure 8.

Photomicrograph of (A) heart of positive control rat (C2) showing branch of coronary arteries (a) has thickened wall and surrounded by increased amount of dense connective tissue (∗). Some endothelial cells protrude into the lumen and others are lost (arrow). (B) Heart of a rat from (E3) received low dose of antioxidants during exposure to CS. Cardiac muscle fibers are widely separated, some of them appear atrophied (arrow) with few inflammatory cells in between (white arrow) (H&E).
Figure 9.
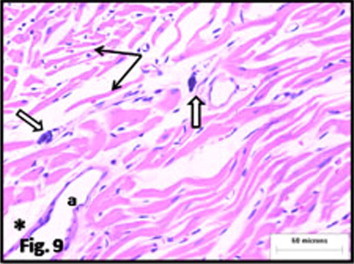
Photomicrograph of heart of rat from (E4) received high dose of antioxidants during exposure to CS. cardiac muscle fibers appear irregularly arranged, widely separated and some are atrophied (arrow). The connective tissue in between shows mast cells (white arrow). A branch of the coronaries (a) appear normal with average amount of connective tissue around it (∗) (H&E).
4. Discussion
The important findings of Poredo et al. (1999) confirmed that smoking adversely affects the vasculature, even on a short-term basis. This study aimed to further study the effect of antioxidants in reversing or decreasing such adverse effects.
This study showed that carotid artery weight was significantly increased in groups exposed to CS; this increase could be attributed to significantly increased IMT of the carotid that was also observed in this study. This was in agreement with Anazawa et al. (2004), who found that exposure of mice to CS for 21 h resulted in increased IMT of the carotid artery.
The administration of antioxidants (vitamin C, vitamin E, and B-carotene) that were used in this study either at low or high doses could reduce carotid artery weight and IMT. These findings were supported by the findings of other researchers; they found that exposure to smoking resulted in increased carotid thickness in male smokers who did not receive any vitamin supplements (De Waart et al., 2000). Other researchers also observed that vitamins E and C shortages exposed smokers to an increased risk of developing vascular diseases (Escolar et al., 1996).
In this study, it was found that exposure to CS resulted in an increase in the heart weight, which could be attributed to the enlargement of the cardiac muscle fibers observed in the histological study. This enlargement might result from pulmonary hypertension induced by exposure to CS. This explanation was adopted by Gvozdjáková et al. (1995), who postulated that long exposure to passive smoking leads to an inhibitory effect on the mitochondrial oxidative phosphorylation, which results in hypertrophy of the cardiac muscles. Török et al. (2000) also found that rabbits exposed to CS for three weeks developed an increase in the catecholamine levels, which was associated with increased contractility and hypertrophy of the cardiac muscle.
During this study, it was observed that exposure to CS resulted in narrowing of lumens and increased the thickness of the walls of most branches of the coronary vessels running among the cardiac fibers. Some cardiac muscles appeared degenerated; others were atrophied. Administration of antioxidants failed to improve changes or return the cardiac weight back to its normal range, as cardiac muscle fibers have no ability for regeneration. On the other hand, branches of the coronaries showed improvement after antioxidant administration.
Exposure to CS significantly increased the cholesterol level in the blood, as observed in this study. These results were in conflict with Abou-Hozaifa and Badr El-Din (1995), who found that the administration of nicotine in drinking water of mice did not result in significant changes in the total cholesterol level. Treatment with antioxidants (either at low or high doses) after stopping of or during exposure to CS decreased the elevated cholesterol level. These findings were supported by those of Al malky et al. (2000), who studied the effect of antioxidants on cholesterol levels in rats, and Porkkala-Sarataho et al. (2000), who studied the effect in humans.
Antioxidants administration also reduced the elevated triglycerides (TG) that resulted from exposure to CS. These results were supported by other studies, which revealed that increased intake of vitamins C and E affects lipid hyper-oxidation and results in the reduction of TG level (Sahin et al. (2002)).
This study revealed that treatment with antioxidants, either during or after stopping exposure to CS, reduced LDL that had been increased as a result of exposure to CS. These results are supported by other studies that have revealed that vitamin E inhibits oxidation of both LDL and VLDL of fasting patients (Rajalakshmi et al., 2000).
Exposure to CS decreases the level of HDL, and stopping smoking increases it, as this study shows. These results are in agreement with those of Maida and Howlett (1990), who noticed that inhalation of CS affects lipoprotein metabolism and decreases the level of HDL. This study confirms that treatment with antioxidants increases HDL. This finding was augmented by the findings of Fotherby et al. (2000), who reported that oral administration of vitamin C to females resulted in increased HDL and decreased LDL levels.
The endothelial cell damage that was noticed in this study could be attributed to the free radicals (NO2, OH, and O2) induced by nicotine and circulated in the blood. This endothelial cell damage, in addition to the oxidative stress induced by smoking, resulted in atherosclerosis. This explanation was adopted by Sarkar et al. (1999) and Lin et al. (1992).
Exposure to CS also resulted in a marked increase in adventitial layer thickness, which showed many lipid-containing cells. These lipids were increased in the blood due to exposure to CS, as revealed by biochemical study.
Treatment with antioxidants during or after stopping the exposure to CS resulted in marked improvement of the structure of the carotid artery. This could be explained by the findings of Panda et al. (2001). They found that vitamin C prevents the damage of nitric oxide caused by superoxide produced during exposure to CS. These findings were confirmed by a previous study, which revealed that the intake of ascorbic acid for prolonged periods by coronary heart disease patients leads to marked improvement due to restoring of the endothelial cell function and dilatation of the coronary artery (Gokce et al. 1999).
In this study, it was observed that improvement in the structure of the carotid artery and the lipid profile in the group that received antioxidants during exposure to CS were better than in the group that received antioxidants after stopping exposure to CS. This could be attributed to the role of these antioxidants in removing the free radicals produced by exposure to CS once they were formed.
Regarding the histological structure of the carotid and heart, it was also observed that the responses to antioxidants given at low doses were better than those given at high doses. On the other hand, administration of high doses of antioxidants improved the lipid profile better than the low doses. It has been noted that high doses of antioxidants should be cautiously given, as they might act as oxidative agents and damage tissues (Rietjens et al., 2002).
In conclusion, these results seemed to indicate that antioxidants ameliorate the CS contribution to atherosclerosis, as measured by carotid IMT and lipid profile. It also indicated that antioxidants could not completely reverse the CS-induced changes after stopping smoking. Simultaneous intake of antioxidants could ameliorate the CS-induced changes apart from those of the heart. Proloned study is recommended to assess the effect of long-term use of these antioxidants on the heart.
References
- Abou-Hozaifa B.M., Badr El-Din N.K. Royal jelly, a possible agent to reduce the nicotine-induced atherogenic lipoprotein profile. Saudi Med. J. 1995;16:337–342. [Google Scholar]
- Al malky, W., Ghllab, E.M., Mahmod, E.M., 2000. Comparative study on effect of natural and synthetic sources of antioxidants(vitamin A, E and C) on serum lipids in hypercholesterolemic rats. Faculty of Agriculture El fayoum. 14, 189–199.
- Anazawa T., Dimayuga P.C., Li H., Tani S., Bradfield J., Chyu K. Effect of exposure to cigarette smoke on carotid artery intimal thickening the role of inducible no synthase. Arterioscler. Thromb. Vasc. Biol. 2004;24:1652–1658. doi: 10.1161/01.ATV.0000139925.84444.ad. [DOI] [PubMed] [Google Scholar]
- Baker D.L., Krol E.S., Jacobsen N., Liebler D.C. Reactions of betacarotene with cigarette smoke oxidants. Identification of carotenoid oxidation products and evaluation of the prooxidant/antioxidant effect. Chem. Res. Toxicol. 1999;12:535–543. doi: 10.1021/tx980263v. [DOI] [PubMed] [Google Scholar]
- Bancroft J., Cook H. Churchill Livingstone; Edinburgh, New York: 1994. Manual of Histological Technique and their Diagnostic Application. p. 457. [Google Scholar]
- Barnoya J., Glantz S.A. Cardiovascular effects of secondhand smoke: nearly as large as smoking. Circulation. 2005;111:2684–2698. doi: 10.1161/CIRCULATIONAHA.104.492215. [DOI] [PubMed] [Google Scholar]
- Bogren H.G., Mohiaddin R.H., Klipstein R.K., Firmin D.N., Underwood R.S., Rees S.R. The function of the aorta in ischemic heart disease: a magnetic resonance and angiographic study of aortic compliance and blood flow patterns. Am. Heart J. 1989;118:234–247. doi: 10.1016/0002-8703(89)90181-6. [DOI] [PubMed] [Google Scholar]
- Bonithon-Kopp C., Coudray C., Berr C., Touboul P.J., Feve J.M., Favier A., Ducimetiere P. Combined effects of lipid peroxidation and antioxidant status on carotid atherosclerosis in a population aged 59–71 y: the EVA study. Am. J. Clin. Nutr. 1997;65:121–127. doi: 10.1093/ajcn/65.1.121. [DOI] [PubMed] [Google Scholar]
- Carr A., Frei B. The role of natural antioxidants in preserving the biological activity of endothelium-derived nitric oxide. Free Radic. Biol. Med. 2000;28:1806–1814. doi: 10.1016/s0891-5849(00)00225-2. [DOI] [PubMed] [Google Scholar]
- Chao J.C., Huang C.H., Wu S.J., Yang S.C., Chang N.C., Shieh M.J. Effects of B-carotene, vitamin C and E on antioxidant status in hyperlipidemic smokers. J. Nutr. Biochem. 2002;13:427–434. doi: 10.1016/s0955-2863(02)00188-2. [DOI] [PubMed] [Google Scholar]
- Conklin D.J., Haberzett L.P., Prough R.A., Bhatnagar A. Glutathione-S-transferase P protects against endothelial dysfunction induced by exposure to tobacco smoke. Am. J. Physiol. Heart Circ. Physiol. 2009;296:H1586–H1597. doi: 10.1152/ajpheart.00867.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Waart F.G., Smilde T.J., Wollersheim H., Stalenhoef A.F.H., Kok F.J. Smoking characteristics, antioxidant vitamins, and carotid artery wall thickness among life-long smokers. J. Clin. Epidemiol. 2000;53:707–714. doi: 10.1016/s0895-4356(99)00198-5. [DOI] [PubMed] [Google Scholar]
- Dutta A., Dutta S.K. Vitamin E and its role in the prevention of atherosclerosis and carcinogenesis: a review. J. Am. Coll. Nutr. 2003;22:258–268. doi: 10.1080/07315724.2003.10719302. [DOI] [PubMed] [Google Scholar]
- Escolar J.D., Martinez M.N., Escolar M.A., Arranz M., Gallego B., Roche P.A. Tobacco smoke and age as risk factors in emphysema. Morphometrical study on the rat. Histol. Histopathol. 1996;1:7–16. [PubMed] [Google Scholar]
- Ezzati, M., Lopez, A.D., 2003. Estimates of global mortality attributable to smoking in Lancet, 362, 847-852. [DOI] [PubMed]
- Fotherby M.D., Williams J.C., Forster L.A., Craner P., Ferns G.A. Effect of vitamin C on ambulatory blood pressure and plasma lipids on older persons. Journal of Hypertension. 2000;189(4):411–415. doi: 10.1097/00004872-200018040-00009. [DOI] [PubMed] [Google Scholar]
- Gokce N., Keaney J.F., Frei B., Holbrook M., Olesiak M., Zachariah B.J. Long-term ascorbic acid administration reverses endothelial vasomotor dysfunction in patients with coronary artery disease. Circulation. 1999;99:3234–3240. doi: 10.1161/01.cir.99.25.3234. [DOI] [PubMed] [Google Scholar]
- Gvozdjáková A., Kucharská J., Herichov I., Koprena I., Gvozdj K.J. On the protective effect of coenzyme Q10 on smoke mitochondrial cardiomyopathy in rabbit. Cor. Vasa. 1995;37:1453–1457. [Google Scholar]
- Heiss G., Sharett A.R., Barnes R., Chambless L.E., Szklo M., Alzola C. Carotid atherosclerosis measured by B-mode ultrasound in populations: associations with cardiovascular risk factors in the ARIC study. Am. J. Epidemiol. 1991;134:250–256. doi: 10.1093/oxfordjournals.aje.a116078. [DOI] [PubMed] [Google Scholar]
- Higenbottam T., Shipley M.J., Rose G. Cigarettes, lung cancer, and coronary heart disease: the effects of inhalation and tar yield. J. Epidemiol. Community Health. 1982;36:113–117. doi: 10.1136/jech.36.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard G., Burke G.L., Szklo M., Tell G.S., Eckfeldt J., Evans G., Heiss G. Active and passive smoking are associated with increased carotid wall thickness. The atherosclerosis risk in communities study. Arch. Intern. Med. 1994;154:1277–1282. [PubMed] [Google Scholar]
- Howard G., Wagenknecht L.E., Burke G.L., Ez-Roux A., Evans G.W., McGovern P. Cigarette smoking and progression of atherosclerosis: the atherosclerosis risk in communities (ARIC) study. JAMA. 1998;279:119–124. doi: 10.1001/jama.279.2.119. [DOI] [PubMed] [Google Scholar]
- Iribarren C., Folsom A.R., Jacobs D.R., Gross M.D., Belcher J.D., Eckfeldt J.H. Association of serum vitamin levels, LDL susceptibility to oxidation, and autoantibodies against MDA-LDL with carotid atherosclerosis. A case control study. Arterioscler. Thromb. Vasc. Biol. 1997;17:1171–1177. doi: 10.1161/01.atv.17.6.1171. [DOI] [PubMed] [Google Scholar]
- Joensuu T., Salonen R., Winblad I., Korpela H., Salonen J.T. Determinants of femoral and carotid artery atherosclerosis. J. Intern. Med. 1994;236:79–84. doi: 10.1111/j.1365-2796.1994.tb01123.x. [DOI] [PubMed] [Google Scholar]
- Kannel W.B., McGee D., Castelli W.P. Latest perspective on cigarette smoking and cardiovascular disease: the Framingham Study. J. Cardiac. Rehabil. 1984;4:267–277. [Google Scholar]
- Kiowski W., Linder L., Stoschitzky K., Pfisterer M., Burckhardt D., Burkart F. Diminished vascular response to inhibition of endothelium-derived nitric oxide and enhanced vasoconstriction to exogenously administered endothelin-1 in clinically healthy smokers. Circulation. 1994;90:27–34. doi: 10.1161/01.cir.90.1.27. [DOI] [PubMed] [Google Scholar]
- Lin S.J., Hong C.Y., Chang M.S., Chiang B.N., Chien S. Long-term nicotine exposure increases aortic endothelial cell death and enhances transendothelial macromolecular transport in rats. Arterioscler. Thromb. 1992;12:1305. doi: 10.1161/01.atv.12.11.1305. [DOI] [PubMed] [Google Scholar]
- Maida V., Howlett G.J. Effects of cigarette smoking and dietary lipids on rat lipoprotein metabolism. Atherosclerosis. 1990;80:209–216. doi: 10.1016/0021-9150(90)90028-h. [DOI] [PubMed] [Google Scholar]
- Paget, G., Barnes, J., 1964. Evaluation of drug activities. In: Laurence and Bacharach (Eds.), Pharmacometrics, vol. 1. Academic Press, New York.
- Panda K., Chattopadhyay R., Ghosh M.K., Chattopadhyay D.J., Chatterjee I.B. Vitamin C prevents cigarette smoke induced oxidative damage of proteins and increased proteolysis. Free Radic. Biol. Med. 1999;27:1064–1079. doi: 10.1016/s0891-5849(99)00154-9. [DOI] [PubMed] [Google Scholar]
- Panda K., Chattopadhyay R., Chattopadhyay D., Chatterjee B. Cigarette smoke-induced protein oxidation and proteolysis is exclusively caused by its tar phase: prevention by vitamin C. Toxicol. Lett. 2001;6:21–32. doi: 10.1016/s0378-4274(01)00376-9. [DOI] [PubMed] [Google Scholar]
- Poredo P., Orehek M., Tratnik E. Smoking is associated with dose-related increase of intima-media thickness and endothelial dysfunction. Angiology. 1999;50:201–208. doi: 10.1177/000331979905000304. [DOI] [PubMed] [Google Scholar]
- Porkkala-Sarataho E., Salonen J.T., Nyyssnen K., Kaikkonen J., Salonen R., Ristonmaa U. Long-term effects of vitamin E, vitamin C, and combined supplementation on urinary 7-hydro-8-oxo-2-deoxyguanosine, serum cholesterol oxidation products, and oxidation resistance of lipids in nondepleted men. Arterioscler. Thromb. Vasc. Biol. 2000;20:2087–2093. doi: 10.1161/01.atv.20.9.2087. [DOI] [PubMed] [Google Scholar]
- Powell J.T., Higman D.J. Smoking, nitric oxide and the endothelium. Br. J. Surg. 1994;81:785–787. doi: 10.1002/bjs.1800810602. [DOI] [PubMed] [Google Scholar]
- Powell J.T., Edwards R.J., Worell P.C., Franks P.J., Greenhalgh R.M., Poulter N.R. Risk factors associated with the development of peripheral arterial disease in smokers: a case control study. Atherosclerosis. 1997;129:41–48. doi: 10.1016/s0021-9150(96)06012-1. [DOI] [PubMed] [Google Scholar]
- Rajalakshmi K., Prema G., Devaraj S.N., Gurumurthi P. Effect of eugenol and tincture of crataegus (TCR) on in vitro oxidation of LDL+VLDL isolated from plasma of non-insulin dependent diabetic patients. Indian J. Exp. Biol. 2000;38(5):509–511. [PubMed] [Google Scholar]
- Rietjens I.M., Boersma M.G., De Haan L., Spenkelink B., Awad H.M., Cnubben N.H.P. The pro-oxidant chemistry of the natural antioxidant vitamin C, vitamin E, carotenoids and flavonoids. Environ. Toxicol. Pharmacol. 2002;11:321–333. doi: 10.1016/s1382-6689(02)00003-0. [DOI] [PubMed] [Google Scholar]
- Sahin K., Kucuk O., Sahin N., Sari M. Effect of vitamin C and vitamin E on lipid peroxidation status, serum hormone, metabolite. And mineral concentrations of Japanese quails reared under heart stress (34C) Int. J. Vitam. Nutr. Res. 2002;72:91–100. doi: 10.1024/0300-9831.72.2.91. [DOI] [PubMed] [Google Scholar]
- Sarkar R., Gelabert H.A., Mohiuddin K.R., Thakor D.K., Santibanes-Gallerani A.S. Effect of cigarette smoke on endothelial regeneration in vivo and nitric oxide levels. J. Surg. Res. 1999;82(1):43–47. doi: 10.1006/jsre.1998.5502. [DOI] [PubMed] [Google Scholar]
- Smith C.J., Fischer T.H. Particulate and vapor phase constituents of cigarette mainstream smoke and risk of myocardial infarction. Atherosclerosis. 2001;158:257–267. doi: 10.1016/s0021-9150(01)00570-6. [DOI] [PubMed] [Google Scholar]
- Tietz, N.W., 1994. Textbook of Clinical Chemistry, P.A. Techniques and Procedures to Minimize Laboratory Infections. Specimen Collection and Storage Recommendations. WB Saunders Co., Philadelphia.
- Török J., Gvozdjáková A., Kucharská J., Balazovjech I., Kyselá S., Simko F., Gvozdják J. Passive smoking impairs endothelium-dependent relaxation of isolated rabbit arteries. Physiol. Res. 2000;49:135–141. [PubMed] [Google Scholar]
- Ueta E., Tadokoro Y., Yamamoto T., Yamane C., Suzuki E., Nanba E. The effect of cigarette smoke exposure and ascorbic acid intake on gene expression of antioxidant enzymes and other related enzymes in the livers and lungs of Shionogi rats with osteogenic disorders. Toxicol. Sci. 2003;73:339–347. doi: 10.1093/toxsci/kfg082. [DOI] [PubMed] [Google Scholar]
- Wagenknecht L.E., Agostino R., Savage P.J., O’Leary D.H., Saad M.F., Haffner S.M. Duration of diabetes and carotid wall thickness: The insulin resistance atherosclerosis study (IRAS) Stroke. 1997;28:999–1005. doi: 10.1161/01.str.28.5.999. [DOI] [PubMed] [Google Scholar]
- Waynforth, B.H., 1988. Experimental and Surgical Technique in the Rat. Academic Press. A Subsidiary of Harcourt Brace Jovanovich, Publishers. London.


