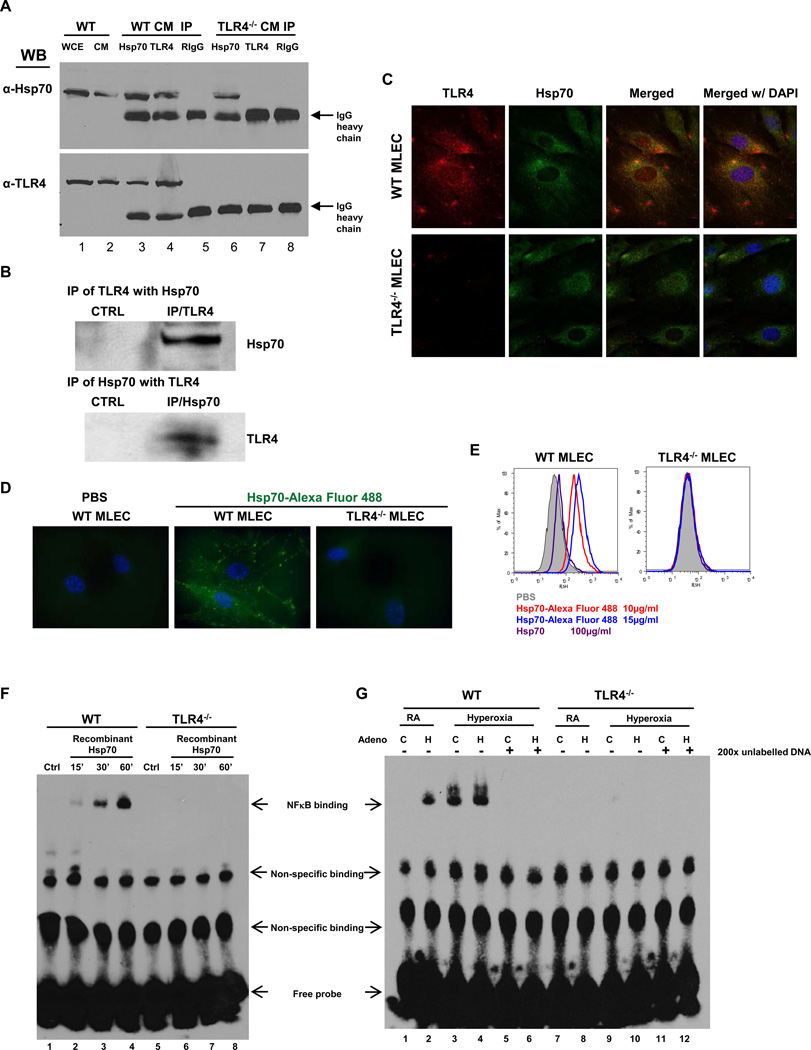
Figure 2. Hsp70 interacts with TLR4 in MLEC and lungs.
A) Immunoprecipitation (IP) of Hsp70 and TLR4 in WT and TLR4−/− MLEC cell membrane (CM). WCE, whole cell extract. RIgG: Rabbit normal IgG. B) IP of TLR4 and Hsp70 with lung lysates. CTRL, control. C) Co-localization of TLR4 and Hsp70 in MLEC using immunofluorescence. WT or TLR4−/− MLEC were immunostained for TLR4 (red) and Hsp70 (green). Nuclei were stained with DAPI (blue) and imaged by confocal microscopy. The left panels show single immunostained images and the rights panels show merged images. Original magnification of all photomicrographs, ×600. D) Recombinant human Hsp70 protein was labeled with Alexa Fluor 488, incubated with WT or TLR4−/− MLEC (10µg/ml) and imaged with laser microscopy. Original magnification of all photomicrographs: ×1000. E) WT or TLR4−/− MLEC were incubated with two concentrations of Alexa 488-labeled recombinant human Hsp70 (10µg/ml and 15µg/ml). Unlabelled Hsp70 protein (100µg/ml) was used to “compete off” labeled Hsp70. Fluorescence intensity was measured by flow cytometry. F) Nuclear extracts prepared from WT and TLR4−/− MLEC incubated with recombinant Hsp70 (10µg/ml) for the indicated time or no treatment (Ctrl) were mixed with biotin-labeled oligonucleotide containing NFκB motif. Bound complexes were analyzed by electrophoresis. The results are representative of at least three independent experiments. G) WT and TLR4−/− MLEC were treated with Ad-Ctrl (C) or Ad-Hsp70 (H) and were exposed to 72h of hyperoxia. RA, room air control. Nuclear extracts were mixed with biotin-labeled oligonucleotide containing NFκB motif. Competitive inhibition of NFκB binding with nonlabeled probe (200×). Bound complexes were analyzed by electrophoresis. The results are representative of at least 3 independent experiments.