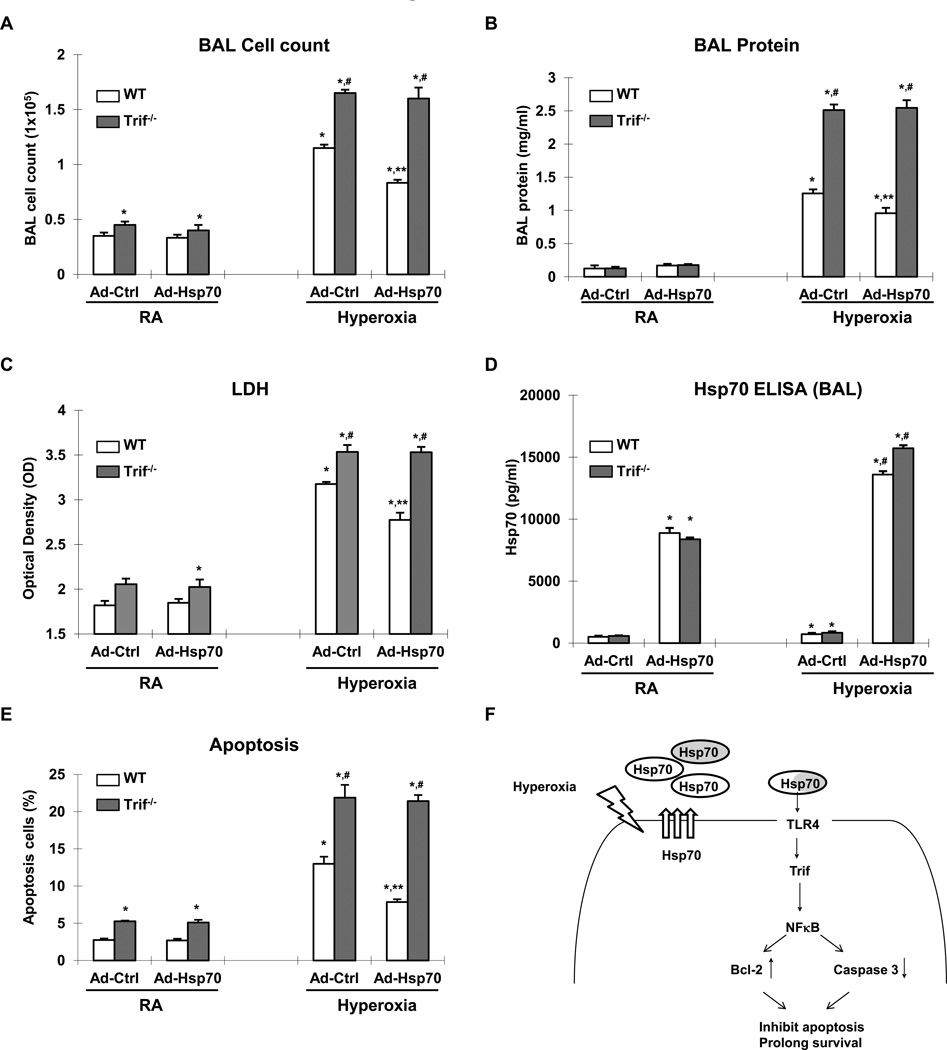
Figure 9. Hsp70-mediated protection is Trif-dependent in mice.
WT or Trif−/− mice treated with Ad-Ctrl or Ad-Hsp70 and were exposed to 72h of hyperoxia. RA, room air control. A) Lung inflammation was detected by BAL cell counts. B) Lung permeability was assessed by BAL protein content. C) LDH activity assay as assessed in BAL fluid. D) Hsp70 ELISA assay in BAL fluid. E) TUNEL staining was performed on lung sections and the number of TUNEL-positive cells were quantitated and expressed as a percentage of the total number of lung cells counted on each section. The values are expressed as mean ± SD. *p<0.01 vs RA WT Ad-Ctrl mice; **p<0.01 vs corresponding Ad-Ctrl mice; #p<0.01 vs corresponding WT mice (n=5 for each group). F) Summary of Hsp70-TLR4 signaling under hyperoxia. We postulate that endothelial TLR4 is required for an adaptive mechanism that delays hyperoxic apoptosis, and prolongs survival. Hyperoxia-induced Hsp70 secretion from MLEC (white circle labeled Hsp70) or other cell type (gray circle labeled Hsp70). Extracellular Hsp70 interacted with TLR4 and transduced signal through Trif-NFκB patyway. Induced expression of Bcl-2 and decreased activation of Caspase 3 inhibit the cell apoptosis and prolong survival.