Abstract
Fifteen spices obtained from common markets were examined for their mould profile. A total of 520 fungal isolates, representing 57 species, were recovered and identified from dried and ground spice samples on three different media using standard dilution plate method. The most heavily contaminated spice samples examined were observed in ginger in order of magnitude of 5325–6800 cfu/g. The most predominant fungal genera encountered were Aspergillus, Penicillium, and Rhizopus. Relative occurrence values of taxa disclosed ranged between 80% for Aspergillusflavus, Aspergillusniger and Penicilliumarenicola, and 10% for some species. Samples obtained from sumac encountered very rare colony counts indicating its antifungal prosperities. The present magnitude of contamination and spectra of mycobiota approximate those reported for similar spice samples. Several potentially mycotoxigenic fungi were isolated from the majority of samples. The present study attracts the attention to potential risk for mycotoxins contamination may be caused as a result of using these spices, especially in great quantities. The study strongly recommends reduction in application of heavily contaminated spices like ginger in food processing and using some others like clove and sumac due to their antimicrobial properties.
Keywords: Spices, Fungi, Mycotoxins, Antimicrobial effect
1. Introduction
Spices and herbs are valued for their distinctive flavors, colors and aromas and are among the most versatile and widely used ingredient in food preparation and processing throughout the world. As with many other agricultural products, spices and herbs may be exposed to a wide range of microbial contamination during pre- and post-harvest. Such contamination may occur during processing storage, distribution, sale and/or use (McKee, 1995). Early cultures also recognized the value of using spices and herbs in preserving foods and for their medicinal value. Spices have been used in many industries, with the food industry and catering being predominant users. Having been dried material from plant origin, spices are commonly heavily contaminated with xerophilic storage moulds and bacteria (Dimić et al., 2000; Romagnoli et al., 2007). Although spices are present in foods in small amounts, they are recognized as important carriers of microbial contamination mainly because of the conditions in which they were grown, harvested and processed. In addition, because of possible neglects during sanitation or processing, foods containing spices are more likely to deteriorate and also could exert harmful effects, having in mind health risks associated with mycotoxins produced by some fungal genera (Koci-Tanackov et al., 2007).
Fungi are the predominant contaminants of spices (Kneifel and Berger, 1994), but most such microbial populations are probably regarded as commensal residents on the plant that survived drying and storage. Soil and air is the main inoculum source for causing contamination in crude spices in field. Other practices like harvesting, handling and packing, cause additional contamination. Moreover, spices are collected in tropical areas by simple methods and are commonly exposed to many contaminants before, being dry enough to prevent microbial growth.
The most frequent fungal contaminants of spices are species from the genera Aspergillus and Penicillium (Silliker et al., 1992; Dimić and Škrinjar, 1995). Some species that belong to these genera are known as potential producers of different toxic substances such as aflatoxins, ochratoxins and sterigmatocystine, i.e. mycotoxins that exhibit toxic, mutagenic, teratogenic and carcinogenic effects in humans and animals (Frisvad et al., 2005; Zinedine et al., 2006).
There are more and more indications that primary liver carcinoma and other serious diseases may be induced by consuming food or using raw materials for food processing contaminated with fungi or mycotoxins. Aflatoxins, ochratoxin A and sterigmatocystin proved resistant to heat and have an ability to accumulate in the organism (Galvano et al., 2005; Jay et al., 2005). Even products stored at low temperatures are vulnerable to some fungi (Duraković et al., 1989). Fungal contamination of spices usually occurs when spices are not properly dried or when stored in a highly humid environment (Dimić et al., 2008).
This paper aims at assessing the intensity and frequency of moulds contamination in common spices in public markets and the potential producers of mycotoxins to highlight their risk assessment.
2. Materials and methods
2.1. Sampling
About 138 samples of 15 different spices were collected randomly from famous supermarkets from Aseer region, Saudi Arabia. Samples (100 g/sample) were collected in sterilized polyethylene bags and stored at −4 °C until use. The names, families and the used parts of each spice are presented in Table 1.
Table 1.
Names, families and the used parted of studied spices.
| No. | English name | Latin name | Family | Used part | Arabic name |
|---|---|---|---|---|---|
| 1 | Cinnamon | Cinnamomumzeylanicum Blume | Lauraceae | Stem bark | Qarfah, Qirfah, Qurfa |
| 2 | Green cumin | Cuminumcyminum L. | Apiaceae | Fruits (frequently called seeds) | Kamoun, Kamun |
| 3 | Shumac, sumac | Rhuscoriaria L. | Anacardiaceae | Dried fruits | Summaaq, Summaq |
| 4 | Ginger | Zingiberofficinale Rosc. | Zingiberaceae | Fleshy rhizome | Zanjabeel, Zanjabil |
| 5 | Indian saffron | Curcumalonga L. | Zingiberaceae | Rhizome | Kurkum, Uqdah safra |
| 6 | Fenugreek | Trigonellafoenum-graecum L. | Fabaceae | Seeds | Hulba, Hilbeh |
| 7 | Black pepper | Pipernigrum L. | Piperaceae | Dried fruits | Fulful, Filfil |
| 8 | Fennel flower | Nigellasativa L. | Ranunculaceae | The deep black, seed grains | Habbah sauda, Habbah al-baraka |
| 9 | Garden thyme | Thymusvulgaris L. | Lamiaceae | Leaves (leaves plus stem) | Satr, Zatr |
| 10 | Cayenne pepper | Capsicumfrutescens L. | Solanaceae | Fruits | Fulful alahmar, Fulful haar |
| 11 | Green cardamom | Elettariacardamomum White et Mason | Zingiberaceae | Seeds | Habbahan, Habbu al-hal |
| 12 | Caraway | Carumcarvi L. | Apiaceae | Fruits | Karaway, Karawiaa |
| 13 | Sweet cumin | Foeniculumvulgare Mill. | Apiaceae | Fruits (usually mistermed seeds) | Shamaar, Shamar |
| 14 | Aniseed | Pimpinellaanisum L. | Apiaceae | Fruits | Habbu al-hulwah, Yansoon |
| 15 | Cloves | Syzygiumaromaticum [L.] Merr. et Perry | Myrtaceae | Buds | Kabsh qarunfil, Kabsh qaranful |
2.2. Mycological studies
Dilution method according to Koch (Harrigan, 1998) was used to determine total fungal counts in spice samples, in triplicates. Ten grams of each sample (fine powder) were added to 90 ml portion of sterile saline solution (0.85%) in 500 ml Erlenmeyer flask and homogenized thoroughly on an electric shaker at constant speed for 30 min. The spice–water suspension was allowed to stand for 10 min with intermittent shaking before being plated. Ten fold serial dilutions were then prepared. One millilitre portion of suitable dilutions was used to inoculate Petri dishes containing 15 ml agar medium fortified by 0.5 mg chloramphenicol/ml medium. Three nutritive media were chosen: Czapek dox agar (dextrose, 10 g/L; NaNO3, 2.0 g/L; KCl, 0.5 g/L; MgSO4·7H2O, 0.5 g/L; FeSO4·7H2O, 0.01 g/L; K2SO4, 0.35 g/L; agar, 15.0 g/L; pH = 6.8 ± 0.2); potato dextrose agar (potatoes infusion, 200 g/L; dextrose, 20 g/L; agar, 15 g/L; pH = 5.6 ± 0.2) and Cooke rose (soytone, 5 g/L; dextrose, 10 g/L; KH2PO4, 1 g/L; MgSO4·7H2O, 0.5 g/L; agar, 20 g/L; rose bengal, 0.035 g/L; pH = 6.0 ± 0.2). Plates were incubated at 28 ± 1 °C for 5–10 days and examined for the growth of moulds. Fung were isolated and identified according to (Raper and Fennel (1977), Domsch et al. (1981), Pitt (1985).
3. Results and discussion
Five hundred and twenty isolates represent 57 species of 23 genera were isolated from 15 spices on the three used media (Tables 2–4). On Czapek medium, 37 species of 17 genera were isolated and identified. Aspergillus, Penicillium and Rhizopus were the most common genera where, they were represented by 10, 7 and 1 species, respectively. Among these species, Aspergillus niger and Aspergillus flavus had the highest occurrence remarks and emerged between 93% and 46.7% of samples, respectively. The most common penicillia were Penicillium arenicola, Penicillium dunkii and Penicillium brevicompactum. They were detected in 26.7–33.3% of samples. Rhizopus was represented only by Rhizopus stolonifer. It contaminated 66.7% of spices samples. Alternaria emerged in 40% of samples and was represented by Alternaria alternata (33.3%) and Alternaria tenuissima (6.7%). Eurotium, Fennellia and Fusarium were detected as moderate contaminating agent where, they were isolated from 26.7% to 33.3% of samples (Table 2). The rest of detected species were encountered as a low or rare contaminating fungi and emerged in less than 20% of samples.
Table 2.
Percentage of samples contaminated by different fungal species isolated from different species Czpek’s medium.
| Fungal species | Cinnamon | Green cumin | Shumac | Ginger | India saffron | Fenugrek | Pepper | Fennel flower | Garden thyme | Cayenne pepper | Green cardamom | Caraway | Sweet cumin | Aniseed | Cloves |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 12 | n = 10 | n = 8 | n = 8 | n = 10 | n = 10 | n = 10 | n = 10 | n = 8 | n = 8 | n = 10 | n = 8 | n = 10 | n = 8 | n = 8 | |
| Acremoniumstrictum | – | – | – | – | – | – | – | – | 25 | – | 20 | – | – | 50 | – |
| Alternariaalternata | – | 40 | – | – | – | – | – | 60 | – | – | – | 50 | 20 | 75 | – |
| Alternariatenuissima | – | – | – | – | – | – | – | – | – | – | – | 50 | – | – | – |
| Aspergillusawamori | – | – | – | – | – | – | – | – | 25 | – | – | – | – | – | – |
| Aspergilluscandidus | – | – | – | – | – | – | 60 | – | – | – | 20 | – | – | – | – |
| Aspergillusclavatus | – | 40 | – | – | 10 | – | – | – | 25 | – | 20 | – | – | – | – |
| Aspergillusflavus | – | – | – | 25 | – | 60 | 20 | 40 | – | 50 | 20 | – | 40 | – | – |
| Aspergillusfumigatus | – | – | – | – | – | 10 | – | – | – | – | – | – | 20 | – | – |
| Aspergillusniger | 83 | 70 | 25 | – | 30 | 80 | 30 | 20 | 50 | 50 | 80 | 67 | 60 | 38 | 25 |
| Aspergillusochraceus | – | – | – | – | – | – | – | – | 25 | – | – | – | – | – | – |
| Aspergillustamarii | – | – | – | 50 | – | – | 10 | – | 25 | – | 20 | – | – | – | – |
| Aspergillusterreus | – | – | – | – | – | 30 | – | – | – | 63 | – | – | – | – | 25 |
| Aspergillussydowii | – | 20 | – | – | – | – | – | – | – | – | – | – | – | 38 | – |
| Aspergillusversicolor | – | – | – | – | – | – | – | 20 | – | – | – | – | – | – | – |
| Cladosporiumcladosporioides | – | 10 | – | – | – | – | – | 40 | – | – | – | – | – | – | – |
| Eurotiumchevalieri | – | – | – | – | – | – | 20 | – | 25 | – | – | – | – | – | – |
| Eurotiumrepens | – | – | – | 25 | 40 | – | 20 | – | – | – | – | – | 40 | – | – |
| Fennellianivea | – | – | – | 25 | – | – | – | – | 25 | – | – | – | – | – | – |
| Fennelliaflavipes | – | – | – | – | – | 20 | – | – | 40 | – | – | – | – | ||
| Fusariumoxysporum | – | 20 | – | – | – | – | – | – | – | – | 20 | – | 20 | – | – |
| Fusariumsubglutinans | – | – | – | – | – | – | – | 20 | – | – | – | – | – | – | – |
| Humicolagrisea | – | – | – | – | – | – | – | – | 25 | – | – | – | – | – | – |
| Macrophominaphaseolina | – | – | – | 25 | – | – | – | – | 25 | – | – | – | – | – | – |
| Mucorracemosus | – | – | – | – | – | – | – | 30 | – | – | – | – | – | 38 | 25 |
| Paecilomyceslilacinus | 17 | – | – | – | – | – | – | – | – | – | – | – | 20 | – | – |
| Penicilliumarenicola | – | – | – | – | 60 | 60 | – | – | 75 | – | – | – | 60 | – | 25 |
| Penicilliumbrevicompactum | – | – | – | – | – | 20 | – | 20 | – | – | 20 | – | – | 38 | – |
| Penicilliumcorylophilum | – | – | – | – | – | – | 20 | – | – | – | – | – | – | – | – |
| Penicilliumdunkii | – | 20 | 25 | 25 | – | – | 20 | – | 25 | – | – | – | – | – | – |
| Penicilliumgriseofulvum | – | – | – | – | – | – | 20 | 20 | – | – | – | – | – | – | – |
| Penicilliumoxalicum | – | 40 | – | – | 60 | – | – | 40 | – | – | – | – | – | – | – |
| Penicilliumwaksmani | – | – | – | 13 | 20 | – | 20 | – | – | – | – | – | – | – | |
| Rhizopusstolonifer | 17 | 20 | 25 | 20 | 80 | 20 | 30 | – | 75 | – | – | 20 | 63 | – | |
| Scopulariopsisbrevicaulis | – | – | – | – | – | – | 10 | – | 25 | – | – | – | – | – | – |
| Stemphyliumbotryosum | – | 60 | – | – | – | – | – | – | 25 | – | – | – | – | 38 | – |
| Trichodermaharzianum | – | – | – | – | – | – | 10 | – | – | – | – | – | – | – | – |
| Ulocladiumchartarum | 17 | – | – | – | – | – | – | 40 | – | – | – | – | – | – | – |
Table 3.
Percentage of samples contaminated by different fungal species isolated from different species coke rose medium.
| Fungal species | Cinnamon | Green cumin | Shumac | Ginger | India saffron | Fenugrek | Pepper | Fennel flower | Garden thyme | Cayenne pepper | Green cardamom | Caraway | Sweet cumin | Aniseed | Cloves |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 12 | n = 10 | n = 8 | n = 8 | n = 10 | n = 10 | n = 10 | n = 10 | n = 8 | n = 8 | n = 10 | n = 8 | n = 10 | n = 8 | n = 8 | |
| Alternariaalternata | – | 40 | – | – | – | – | – | 50 | – | – | – | – | 20 | 75 | – |
| Aspergillusawamori | – | – | – | – | – | – | 20 | – | 25 | – | – | – | 20 | 25 | – |
| Aspergilluscandidus | – | – | – | – | – | 40 | 40 | – | – | 63 | – | – | 20 | – | – |
| Aspergillusflavus | 17 | 60 | – | 25 | 20 | 80 | 40 | 40 | – | 75 | 20 | – | 40 | 25 | 25 |
| Aspergillusniger | 100 | 30 | 38 | 50 | 60 | 60 | 80 | 40 | 50 | 75 | 60 | – | 80 | 50 | – |
| Aspergillusochraceus | – | 20 | – | 50 | – | 20 | 10 | – | 50 | – | – | – | – | – | – |
| Aspergillusterreus | – | – | – | – | – | 20 | – | – | – | 50 | – | – | – | – | 25 |
| Cladosporiumcladosporioides | – | – | – | – | – | – | – | 20 | – | – | – | – | – | 75 | 13 |
| Eurotiumamstelodami | – | – | – | – | – | – | – | – | 25 | – | – | – | – | – | – |
| Eurotiumrepens | – | – | – | 13 | 40 | – | – | – | 25 | – | – | – | 40 | – | – |
| Fennelliaflavipes | – | – | – | – | – | 20 | – | – | – | 63 | – | – | – | 75 | – |
| Fennellia nivea | – | – | – | 25 | – | – | – | – | 25 | – | – | – | – | – | – |
| Fusariumdimerum | – | – | – | – | – | 20 | – | – | – | – | – | – | – | – | |
| Fusariummoniliforme | – | – | – | – | – | – | – | – | – | – | 20 | – | – | – | – |
| Fusariumoxysporum | – | 20 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Gliocladiumroseum | – | 20 | – | – | – | – | – | 20 | – | – | – | – | – | – | – |
| Humicolagrisea | – | – | – | – | – | – | – | – | 38 | – | – | – | – | – | – |
| Mucorcircinelloids | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 25 |
| Mucorheimalis | – | – | – | – | – | – | – | – | 25 | – | – | – | 20 | – | – |
| Mucorracemosus | – | – | – | – | – | – | – | 20 | – | – | – | – | – | – | – |
| Myrotheciumverucaria | – | – | – | – | – | – | – | 20 | – | – | – | – | – | – | – |
| Paecilomyceslilacinus | – | – | – | – | 10 | – | – | – | – | – | – | – | 20 | – | – |
| Phomaherbarum | – | – | – | – | – | – | – | – | – | – | 20 | – | – | – | – |
| Penicilliumdecumbens | – | – | – | – | – | – | – | – | 25 | – | – | – | – | 38 | – |
| Penicilliumarenicola | – | – | – | – | 80 | 60 | 20 | 40 | 75 | – | – | – | 80 | – | 25 |
| Penicilliumaurantigriseum | – | – | – | – | – | – | – | 20 | – | – | – | – | – | – | – |
| Penicilliumbrevicompactum | – | 20 | – | – | – | 20 | – | 20 | – | – | 60 | – | 20 | 50 | – |
| Penicilliumchrysogenum | – | – | – | – | – | 20 | 10 | – | – | – | – | – | – | – | – |
| Penicilliumdiversum | – | – | – | – | – | – | – | 30 | – | – | – | – | – | – | – |
| Penicilliumdunkii | – | 20 | – | 75 | – | – | 40 | – | 25 | – | – | – | – | – | – |
| Penicilliumfuniculosum | – | – | – | 25 | – | – | 10 | 20 | – | – | – | – | 40 | – | – |
| Penicilliumgriseofulvum | – | – | – | – | – | – | – | – | 25 | – | – | – | – | – | – |
| Penicilliumislandicum | – | – | – | – | – | – | – | 10 | – | – | – | – | – | – | – |
| Penicilliumjanthinellum | – | – | – | – | – | – | – | – | – | – | – | 63 | – | – | – |
| Penicilliumoxalicum | – | 40 | – | – | 60 | 20 | – | 40 | – | – | – | – | – | – | – |
| Penicilliumwaksmani | – | 20 | – | 50 | – | 20 | – | – | – | – | – | 50 | – | – | – |
| Rhizopusstolonifer | 17 | 20 | 13 | 25 | 20 | 40 | 40 | 20 | 75 | 38 | 20 | – | 20 | 50 | – |
| Stemphyliumbotryosum | – | 60 | – | – | – | – | – | – | 38 | – | – | – | – | 38 | – |
Table 4.
Percentage of samples contaminated by different fungal species isolated from different species PDA medium.
| Fungal species | Cinnamon | Green cumin | Shumac | Ginger | India saffron | Fenugrek | Pepper | Fennel flower | Garden thyme | Cayenne pepper | Green cardamom | Caraway | Sweet cumin | Aniseed | Cloves |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 12 | n = 10 | n = 8 | n = 8 | n = 10 | n = 10 | n = 10 | n = 10 | n = 8 | n = 8 | n = 10 | n = 8 | n = 10 | n = 8 | n = 8 | |
| Acremoniumstrictum | – | 20 | – | – | – | – | – | – | 25 | – | – | – | – | – | – |
| Alternariaalternata | – | 80 | – | – | – | – | – | 60 | – | – | – | 63 | 20 | 50 | – |
| Aspergillusawamori | – | – | – | 25 | – | – | 40 | – | 25 | – | – | – | – | – | – |
| Aspergillusflavus Link | 33 | 40 | – | – | 20 | 60 | 40 | 20 | – | 25 | 40 | – | 20 | – | – |
| Aspergillusfumigatus | – | 10 | – | – | – | – | – | – | – | – | – | – | 20 | – | – |
| Aspergillusniger | 75 | 80 | 25 | 75 | 40 | 60 | 40 | 60 | 50 | 25 | 80 | 25 | 80 | 50 | – |
| Aspergillusochraceus | – | – | – | – | – | – | 30 | – | 25 | – | – | – | – | – | – |
| Aspergillustamarii | – | – | – | – | – | – | 20 | – | 13 | – | – | – | 20 | 38 | – |
| Aspergillusterreus | – | – | – | – | – | 30 | – | – | – | 25 | – | – | – | – | 25 |
| Aspergillussydowii | 17 | 30 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Aspergillusustus | – | 30 | – | – | – | 20 | – | – | 25 | – | – | – | – | – | – |
| Aspergillusversicolor | – | – | – | – | – | – | – | 20 | – | – | – | – | – | – | – |
| Chaetomiumglobosum | – | – | – | – | – | – | – | – | – | 13 | – | – | – | 38 | – |
| Cladosporiumcladosporioides | – | – | – | – | – | – | – | 20 | – | – | 20 | – | – | 38 | – |
| Cochliobolusspicifer | – | – | – | – | – | – | – | – | 25 | – | – | – | – | 38 | – |
| Eurotiumrepens | – | 30 | – | 38 | – | – | 30 | – | – | – | – | – | 40 | – | – |
| Fennellianivea | – | – | – | 25 | – | – | – | – | – | – | – | – | – | – | – |
| Fennelliaflavipes | – | – | – | – | – | 40 | – | – | 13 | 25 | – | – | – | – | – |
| Fusariumoxysporum | – | 60 | – | – | 20 | – | – | – | – | – | 20 | – | 20 | 75 | – |
| Fusariumsubglutinans | – | – | – | – | – | – | – | 20 | – | – | 20 | 50 | – | – | – |
| Humicolagrisea | – | – | – | – | – | – | – | – | 25 | – | – | – | – | – | – |
| Macrophominaphaseolina | – | – | – | – | – | – | – | – | – | – | 20 | – | – | – | – |
| Mucorracemosus | – | 20 | – | – | – | – | – | 20 | – | – | – | – | – | 38 | 38 |
| Myrotheciumverucaria | – | – | – | – | – | – | – | 20 | – | – | – | – | – | – | – |
| Paecilomycesvarriotii | – | – | – | – | – | 40 | – | – | – | – | – | – | – | – | – |
| Paecilomyceslilacinus | 17 | – | – | – | – | – | 20 | – | – | – | – | – | 20 | – | – |
| Phomaherbarum | – | – | – | – | – | – | – | – | 25 | – | 20 | – | – | – | – |
| Penicilliumarenicola | – | – | – | – | 60 | 60 | 20 | 40 | 75 | – | – | – | 60 | 38 | 25 |
| Penicilliumbrevicompactum | – | – | – | – | – | – | – | 20 | – | – | 60 | 38 | 20 | 25 | – |
| Penicilliumcorylophilum | – | – | – | – | – | 20 | 20 | – | – | – | – | – | 20 | 38 | – |
| Penicilliumdunkii | – | 20 | – | – | – | – | 30 | 10 | – | – | – | – | – | – | – |
| Penicilliumfuniculosum | 8 | – | – | – | – | – | 20 | 20 | – | – | – | – | 20 | – | – |
| Penicilliumoxalicum | 17 | 40 | – | – | 60 | 40 | 20 | 40 | – | – | – | 25 | – | – | – |
| Penicilliumwaksmani | 17 | 20 | – | 38 | – | 20 | – | – | – | – | – | – | – | – | – |
| Rhizoctoniasolani | – | 20 | – | 38 | – | – | – | – | – | – | – | – | – | – | – |
| Rhizopusstolonifer | 17 | – | 25 | 38 | 20 | 60 | 10 | 20 | 50 | 25 | 40 | – | – | – | – |
| Scopulariopsisbrevicaulis | – | – | – | – | – | – | 10 | – | – | – | – | – | – | – | – |
| Stemphyliumbotryosum | – | 40 | – | – | – | – | – | 25 | – | – | – | – | 63 | – | |
| Trichodermaharzianum | – | – | – | – | – | – | 10 | – | – | – | – | – | – | – | – |
| Ulocladiumbotrytis | – | – | – | – | – | – | 40 | – | – | – | – | – | – | – | – |
On Cooke rose medium, there was a basic similarity in species structure and degree of contamination to those obtained on Czapek medium. Sixteen genera including 38 species were isolated and identified. The most common genera were Aspergillus (6 species), Penicillium (12 species) and Rhizopus (1 species). A. niger polluted 86.7% of the samples followed by A. flavus (80%) and Aspergillus ochraceus (33.3%), however, the other three Aspragelli contaminated less than 27% of the samples. Among Penicillium species, both P. arenicola and P. brevicompactum were encountered in 46.7% and 40% of the samples. P. dunkii, Penicillium funiculosum, Penicillium oxalicum and Penicillium waksmani were detected in 26.7% of the samples. The other six penicillin were observed rarely in less than 13.3%. R. stolonifer contaminated 86.7% of the samples. Other genera and species were detected in less than 26.7% of the samples (Table 3).
The same genera in the same sequence which were detected on the two above mentioned media were also isolated on PDA (Table 4). Aspergillus was represented by 10 species and Penicillium was represented by 7 species, however, Rhizopus was represented by only 1 species. A. niger polluted 93.3% of spices samples and A. flavus polluted 60% of them. The other eight aspergilli were detected in less than 26.7%. P. arenicola and P. oxalicum were the common penicillin where, they isolated from 53.3% and 46.7% of the samples, respectively. R. stolonifer contaminated 66.7% of spices. The other fungi had moderate to rare occurrence remark and were isolated from less than 33.3% of the spices (Table 4).
The emergence of aspergilli, penicillin and Rhizopus on the three different media greatly indicates the presence of these fungi as the dominant mycoflora of different spices. This observation was greatly in agreement with other investigators who dealt with mycoflora of spices and medicinal plants (El-Kady et al., 1995; Dimić et al., 2008; Bugno et al., 2006). Early, Takatori et al. (1977) and Ayres et al. (1980) found the Aspergillus and Penicillium spp. the main components of cardamom, cinnamon, fennel, coriander, cumin, black cumin and white pepper. Misra (1981) and Roy et al. (1988) isolated A. flavus, A. niger, Aspergillus fumigatus, A. ochraceus, Aspergillus candidus, Aspergillus sydowii, Chaetomium dolicholrichum, Fusarium verticillioides, P. oxalicum, Alternaria, Curvularia and Rhizopus from the seeds of Amomum subulatum, Coriandrum sativum, Cuminum cyminum, Foeniculum vulgare, Piper nigrum, Cinnamomum zeylanicum, and from the bark of Acacia catechu. Ath-Har et al. (1988) reported that A. flavus, A. niger, Aspergillus nidulans, A. sydowii, A. ochraceus, Penicillium and Rhizopus spp. were most frequently isolated from Capsicum frutescens and other spices. A. flavus, A. candidus, A. niger, Aspergillus luchuensis, A. ochraceus, A. nidulans, Fusarium moniliforme, Fusarium oxysporum, A. alternata, Curvularia spp., Chaetomium spp., Penicillium citrinum, and R. stolonifer were reported as the most common fungi isolated from drug plants (Ayres et al., 1980; Aziz and Youssef, 1991). The most contaminating fungi of black pepper were A. flavus, A. ochraceus and Aspergillus versicolor (Christensen, 1975). The contamination with fungal species resulted from neutral extraneous contamination by dust following storage in humid conditions (Domsch et al., 1981). Fungi fall into two ecological categories, e.g., field and storage fungi. Field fungi were observed to invade developing or mature seeds while it is on the plant, the major field fungi genera are, Alternaria, Fusarium and Cladosporium. On the other hand, storage moulds are those encountered on plants at moisture conditions routinely found in stored products, these fungi are principally species of Aspergillus and Penicillium (Abou Donia, 2008). The spices could be subjected for contamination with fungi mainly during spice processing, storage and transport (Dimić et al., 2008).
In our study, most of the identified fungi have been reported to have the ability to produce mycotoxins (Bugno et al., 2006). In this context, Aziz (1987) found that, A. flavus, Aspergillus parasiticus and Aspergillus oryzae were aflatoxin producers, whereas, A. ochraceus, Penicillium viridicatum and Penicillium variable were ochratoxin A producers. In addition, P. viridicatum, Penicillium chrysogenum and Penicillium commune were penicillic acid producers. According to Pohland and Wood (1987), 70–80% of the penicillia are potential mycotoxins producer (citrinin, patulin, cyclopiazonic acid and peniterm). These results showed that a potential risk for mycotoxins contamination may be caused as a result of using these spices, especially in great quantities. Rani and Singh (1990) found that 89% of samples of fennel, coriander and cumin were contaminated with aflatoxin B1 at the levels 3000, 1640 and 1580 mg/kg, respectively. In addition, Roy et al. (1988) and Roy and Chourasia (1990) determined that the seeds of P. nigrum and Mucuna prurians, and the barks of A. catechu, C. sativum, and Elettaria cardamomun were contaminated with aflatoxin B1 at levels below 20 mg/kg. Aziz and Youssef (1991) isolated A. flavus and A. parasiticus with a high tendency for aflatoxin production from some common herbal drugs and spices. Aziz et al. (1998) studied contamination of some common medicinal plant samples and spices and their mycotoxins. Ten fungal genera viz. A. flavus, A. parasiticus, A. niger, F. oxysporum and P. viridicatum occurred most often on the medicinal plant samples. Direct determination of mycotoxins in medicinal plant samples revealed aflatoxin B1 in 17 samples at an average of from 10 to 160 mg/kg, ochratoxin A in three samples at an average of from 20 to 80 mg/kg, and no detection of penicillic acid, zearalenone or T-toxin. Karan et al. (2005) found ochratoxin A in concentration range of 26–33 μg/kg in allspice, oregano and hot pepper but did not prove the presence of aflatoxin B1 and G1. Beside aflatoxin detected in 16 samples of black pepper, fennel, caraway, marjoram, dill and allspice in concentration range 8–35 μg/kg, El-Kady et al. (1995) also found 10–23 μg/kg sterigmatocystin in ten samples of paprika, caraway and marjoram.
A literature review on the incidence of mycotoxins as contaminants of various seasonings indicated the presence of aflatoxins (Vrabcheva, 2000), which are more frequently found in red peppers (paprika, chilly and capsicum), nutmeg, mustard and ginger. High concentrations of aflatoxins are frequently detected in nutmeg, particularly, aflatoxins B1 and B2. Freire et al. (2000) isolated a wide range of field and storage fungi, totaling 42 species from black pepper, white pepper and Brazil nut. Chaetocin, penitrem A and xanthocillin were identified only from black pepper, while tenuazonic acid was identified from both black and white pepper. Aflatoxin G2, chaetoglobosin C and spinulosin were identified from poor quality Brazil nuts. Leistner and Pitt (1977) found that, out of 442 Penicillium isolates, 44 synthesized penicillic acid, 17 ochratoxin A, 11 penitrem, 10 citrinin, 6 patulin and 3 produced both patulin and citrinin. Overy and Frisvad (2005) studied the mycotoxin production and post-harvest storage rot of ginger (Zingiber officinale). He found that P. brevicompactum to be the predominant species isolated from 85% of the samples. Mycophenolic acid was identified from corresponding tissue extracts. Aflatoxin production at various stages of development in cardamom and black pepper was reported by Banerjee et al. (1993). The toxin was assessed using MAB-based ELISA. All A. flavus isolates tested produced aflatoxin B1 in amounts ranging from 65 to 3000 ng/ml. In another study by Geetha and Reddy (1990), A. flavus was indicated in the production of carcinogenic aflatoxin, mainly in ginger, mustard, garlic and pepper. The highest fungal counts were observed in black pepper and the lowest in curry leaves. When three spices – corianders, fennel and ginger collected from Bihar were screened for aflatoxin producing fungi, A. flavus predominated and most isolates produced only aflatoxin B1 in varying amounts (Prasad et al., 1984). Cooking experiments showed that aflatoxin levels in spiced sauces are not reduced by domestic cooking with either microwave or conventional gas oven heating (Macdonald and Castle, 1996). Klieber (2001) studied the aflatoxin contamination level in various chilly products like chilly powder, paprika spices, dried fruit and sauces in retail stores in Adelaide, Australia and its management.
Our results revealed that, the fifteen investigated spices could be grouped into three categories based on their affinity to be contaminated with moulds (Fig. 1). The first groups include spices which produced > 1000 cfu/g and were considered to have a high affinity to contamination. This group included ginger, fenugreek, fennel, garden thyme, red pepper, sweet cumin and aniseed. The highest contaminated spice was ginger (5325–6800 cfu/g) (Fig. 2), however, the other six spices were relatively low contaminated with fungi (1010–2300 cfu/g). The second group was moderately contaminated with fungi (100–1000 cfu/g). Cinnamon, green cumin, pepper, green cardamom, caraway and cloves were belonged to this category. Cloves were the lowest contaminated spice in this group and had 163–300 cfu/g (Fig. 3). This could be due to antimicrobial properties of cloves and its essential oils that are highly effective against moulds (Neilsen and Rios, 2000; Guyenot et al., 2003). The third group was considered as very low contaminated spices and produced <100 cfu/g (Fig. 4). Sumac was the only spice contained in this group and had 50–63 100 cfu/g. Incidence of various mycobiota on the examined spice samples appear to be comparable to or lower than those reported on spices from other countries (Aziz et al., 1998; Elshafie et al., 2002). The present study proves antifungal effect of sumac and its low affinity to be contaminated with moulds. The antimicrobial activity of sumac extract against the Gram-positive microorganisms was reported by other investigators (Nasar-Abbasa and Kadir Halkman, 2004).
Figure 1.
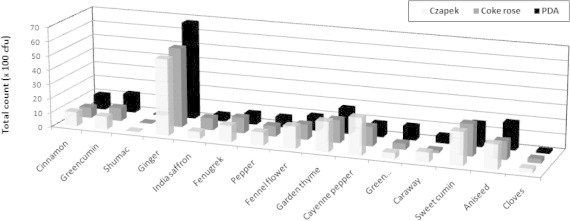
Total count of detected fungi in spices on different growth media.
Figure 2.
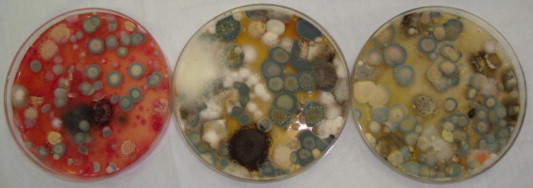
Heavy contaminated spice with fungi (ginger, 5325–6800 cfu g−1) on different media, coke rose (left), PDA (middle) and Czapek (right).
Figure 3.
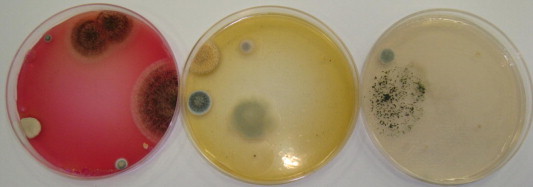
Low contaminated spice with fungi (cloves, 163–300 cfu g−1) on different media, coke rose (left), PDA (middle) and Czapek (right).
Figure 4.
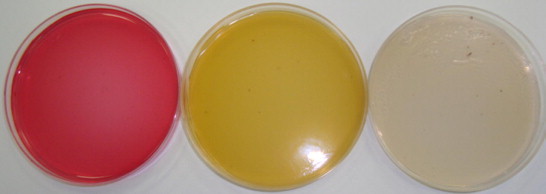
No fungal contamination appears from sumac indicating its antifungal properties on the three media.
References
- Abou Donia M.A. Microbiological quality and aflatoxinogenesis of Egyptian spices and medicinal plants. Global Vet. 2008;2(4):175–181. [Google Scholar]
- Ath-Har M.A., Prakash H.S., Shetty H.S. Mycoflora of Indian spices with special reference to aflatoxin producing isolates of Aspergillusflavus. Indian J. Microbiol. 1988;28:125–127. [Google Scholar]
- Ayres, G.I., Mund, T.I., Sondin, E.W., 1980. Microbiology of Food Spices and Condiments. A Series of Books in Food and Nutrition. Schmeigert, 249pp.
- Aziz, N.H., 1987. Etiology of Toxin-producing Fungi from the Class of Deuteromycetes Occurring in Various Feed Products. Ph.D. Thesis, Agricultural University, Cracow, Poland.
- Aziz N.H., Youssef Y.A. Occurrence of aflatoxins and aflatoxins-producing moulds in fresh and processed meal in Egypt. Food Addit. Contam. 1991;3:321–331. doi: 10.1080/02652039109373981. [DOI] [PubMed] [Google Scholar]
- Aziz N.H., Youssef Y.A., El-Fouly M.Z., Moussa L.A. Contamination of some common medicinal plant samples and spices by fungi and their mycotoxins. Bot. Bull. Acad. Sin. 1998;39:279–285. [Google Scholar]
- Banerjee A., Mathews R.P., Prakash S.H., Shetty H.S. Mycobiotic and toxigenic Aspergillusflavus associated with developing cardamom and pepper. Mycol. Res. 1993;97:1403–1406. [Google Scholar]
- Bugno A., Buzzo Almodovar A.A., Caldas Pereira T., de Jesus Andreoli Pinto T., Sabino M. Occurrence of toxigenic fungi in herbal drugs. Braz. J. Microbiol. 2006;37(1):317–326. [Google Scholar]
- Christensen C.M. University of Minnesota Press; Minneapolis: 1975. Moulds, Mushrooms and Mycotoxins. 26pp. [Google Scholar]
- Dimić G., Škrinjar M. Toksigene plesni i miktoksini u za_inskim smešama I biberu u zrnu koriš_enim u industriji mesa. Tehnologija mesa. 1995;5:302–305. [Google Scholar]
- Dimić G., Škrinjar M., Došen-Bogićević V. Plesni, potencijalni proizvoďači sterigmatocistina u začinima. Tehnologija mesa. 2000;41(4–6):131–137. [Google Scholar]
- Dimić G.R., Kocić-Tanackov S.D., Tepić A.N., Vujičić B.L., Šumić Z.M. Mycopopulation of spices. BIBLID. 2008;39:1–9. [Google Scholar]
- Domsch K.H., Gams W., Anderson T.H. vols. 1 and 2. Academic Press; London: 1981. (Compendium of Soil Fungi). [Google Scholar]
- Duraković S., Galić J., Pajnović P. Toksični i kancerogeni metaboliti gljiva u namirnicama i krmivima. Hrana i ishrana. 1989;2:71–100. [Google Scholar]
- El-Kady I.A., El-Maraghy S.M., Mostafa M.E. Natural occurrence of mycotoxins in different spices in Egypt. Folia Microbiologic. 1995;40(3):297–300. doi: 10.1007/BF02814212. [DOI] [PubMed] [Google Scholar]
- Elshafie E.A., Al-Rashdi T.A., Al-Bahry S.N., Bakheit C.S. Fungi and aflatoxins associated with spices in the Sultanate of Oman. Mycopathologia. 2002;155(3):155–160. doi: 10.1023/a:1020427527963. [DOI] [PubMed] [Google Scholar]
- Freire F.D.O., Kozakiewicz Z., Paterson R.R.M. Mycoflora and mycotoxins in Brazilian black pepper, white pepper and Brazil nuts. Mycopathologia. 2000;149:13–19. doi: 10.1023/a:1007241827937. [DOI] [PubMed] [Google Scholar]
- Frisvad C.J., Skouboe P., Samson A.R. Taxonomic comparison of three different groups of aflatoxin producers and new efficient producer of aflatoxin B1, sterigmatocystine and 3-O-methylsterigmatocystine, Aspergillusrumbelli sp. nov. Syst. Appl. Microbiol. 2005;28:442–453. doi: 10.1016/j.syapm.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Galvano F., Ritieni A., Piva G., Pietri A. Mycotoxins in the human food chain. In: Duarate Diaz, editor. The Mycotoxin Blue Book. Nottingham University Press; England: 2005. pp. 187–225. [Google Scholar]
- Geetha G.S., Reddy T.K.R. Aspergillusflavus link and its occurrence in relation to other mycoflora on stored spices. J. Stored Prod. Res. 1990;26:211–213. [Google Scholar]
- Guyenot M.E., Ramos A.J., Seto L., Purroy P., Sanchis V., Marin S. Antifungal activity of volatile compounds generated by essential oils against fungi commonly causing deterioration of bakery products. J. Appl. Microbiol. 2003;94:893–899. doi: 10.1046/j.1365-2672.2003.01927.x. [DOI] [PubMed] [Google Scholar]
- Harrigan, W., 1998. Laboratory Methods in Food Microbiology. Academic Press, San Diego, pp. 359–375.
- Jay, J., Loessner, M., Golden, D., 2005. In: Dennis R. Heldman (Ed.), Modern Food Microbiology. Springer Science + Business Media, Inc., New York, USA, pp. 709–726.
- Karan D., Vukojević J., Milićević D., Ljajević-Grbić M., Janković V. Kontrola prisustva plesni i mikotoksina u pojedinim začinima koji se koriste u industriji mesa. Tehnologija mesa. 2005;46(5–6):306–310. [Google Scholar]
- Klieber A. Aflatoxin contamination and its management in chilli and paprika products in Australia. Food Aust. 2001;53:90–92. [Google Scholar]
- Kneifel W., Berger E. Microbial criteria of random samples of spices and herbs retailed on the Austrian market. J. Food Prot. 1994;57:893–901. doi: 10.4315/0362-028X-57.10.893. [DOI] [PubMed] [Google Scholar]
- Koci-Tanackov S.D., Dimi G.R., Karali D. Contamination of spices with moulds potential producers of sterigmatocystine. APTEFF. 2007;38:29–35. [Google Scholar]
- Leistner L., Pitt J.I. Miscellaneous Penicillium toxins. In: Rodricks J.V., Hesseltine C.V., Mehlman A.M., editors. Mycotoxins and Human and Animal Health. Pathotox Publishers, Park Forest South; Illinois: 1977. pp. 639–653. [Google Scholar]
- Macdonald S., Castle L. A UK retail survey of aflatoxin in herbs and spices and their fate during cooking. Food Addit. Contam. 1996;13:121–128. doi: 10.1080/02652039609374387. [DOI] [PubMed] [Google Scholar]
- McKee L.H. Microbial contamination of spices and herbs: a review. Lebensm. Wiss. Technol. 1995;28:1–11. [Google Scholar]
- Misra N. Influence of temperature and relative humidity on fungal flora of some spices in storage. Z. Lebensm. Unters. Forsch. 1981;172(1):30–31. [Google Scholar]
- Nasar-Abbasa S.M., Kadir Halkman A. Antimicrobial effect of water extract of sumac (Rhuscoriaria L.) on the growth of some food borne bacteria including pathogens. Int. J. Food Microbiol. 2004;97:63–69. doi: 10.1016/j.ijfoodmicro.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Neilsen V.P., Rios R. Inhibition on fungal growth on bread, by volatile components from spices and herbs, and the possible application in active packaging, with special emphasis on mustard essential oil. Int. J. Food Microbiol. 2000;60:219–229. doi: 10.1016/s0168-1605(00)00343-3. [DOI] [PubMed] [Google Scholar]
- Overy D.P., Frisvad J.C. Mycotoxin production and post-harvest storage rot of ginger (Zingiberofficinale) by Penicilliumbrevicompactum. J. Food Prot. 2005;68:607–609. doi: 10.4315/0362-028x-68.3.607. [DOI] [PubMed] [Google Scholar]
- Pitt, J.I., 1985. A Laboratory Guide to Common Penicillium species. Commonwealth Mycological Institute, Kew, Surrey, England, p. 184.
- Pohland A.E., Wood G.E. Occurrence of mycotoxins in food. In: Krogh P., editor. Mycotoxins in Food. Academic Press; London, UK: 1987. pp. 35–64. [Google Scholar]
- Prasad T., Bilgrami K.S., Thakur M.K., Anjana S. Aflatoxin problem in some common spices. J. Indian Bot. Soc. 1984;63:171–173. [Google Scholar]
- Rani, N., Singh, S., 1990. Aflatoxin contamination of some umbelliferous spices of human use. In: Int. Symp. and Workshop on Food Cont. Mycotoxins and Phycotoxins, November 4–15, Cairo, Egypt, 1990, pp. 79–80 (Abst. Book).
- Raper K.P., Fennel D.I. R.E. Krieger Publishing Company; Huntington, New York: 1977. “The Genus Aspergillus”. [Google Scholar]
- Romagnoli B., Menna V., Gruppioni N., Bergamini C. Aflatoxins in spices, aromatic herbs, herbs–teas and medicinal plants marketed in Italy. Food Control. 2007;18:697–701. [Google Scholar]
- Roy A.K., Chourasia H.K. Mycoflora, mycotoxin producibility and mycotoxins in traditional herbal drugs from India. J. Gen. Appl. Microbiol. 1990;36:295–302. [Google Scholar]
- Roy A.K., Sinha K.K., Chourasia H.K. Aflatoxin contamination of some common drug plants. Appl. Environ. Microbiol. 1988;54:842–843. doi: 10.1128/aem.54.3.842-843.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silliker J.H., Elliot R.P., Bairol-Parker A.C., Bryan F.L., Christian J.H.B., Clark D.S., Olson J.C., Roberts T.A., Jr. vol. II. Academic Press; New York, London: 1992. (Microbial Ecology of Foods). [Google Scholar]
- Takatori K., Watanabe K., Udagawa S., Kurata H. Mycoflora of imported spices and inhibitory effects of the spices on the growth of some fungi. Proc. Jpn. Assoc. Mycotoxins. 1977;9:36–38. [Google Scholar]
- Vrabcheva T.M. Mycotoxins in spices. Vopr. Pitan. 2000;69:40–43. [PubMed] [Google Scholar]
- Zinedine A., Brera C., Elakhdari S., Catano C., Debegnach F., Angelini S., De Santis B., Faid M., Benlemlih M., Minardi V., Miraglia M. Natural occurrence of mycotoxins in cereals and spices commercialized in Morocco. Food Control. 2006;17:868–874. [Google Scholar]


