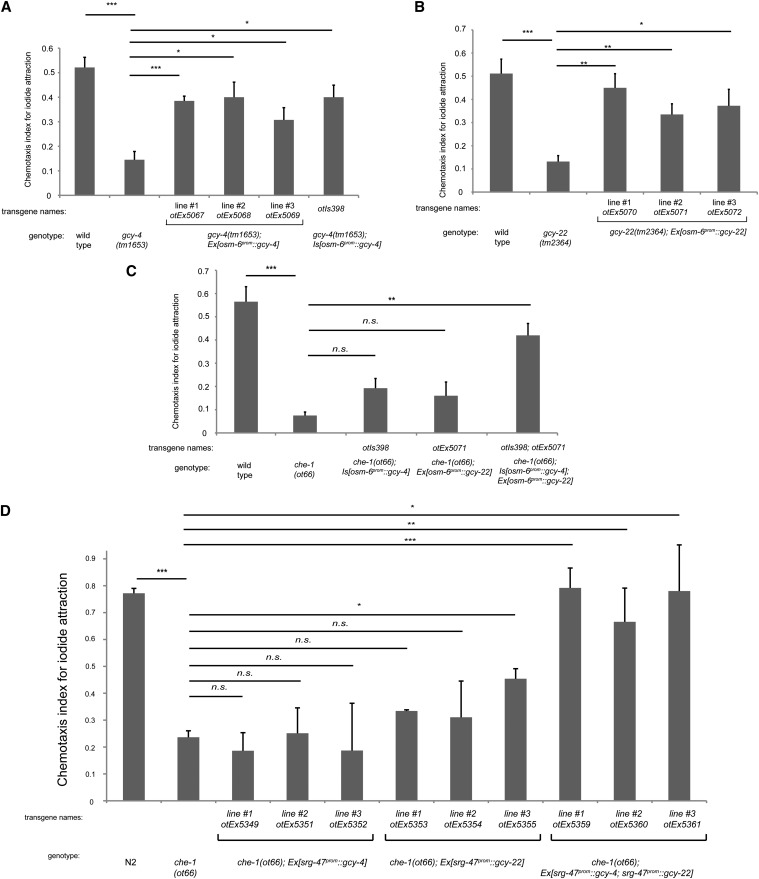
Figure 5.
Ectopic expression of gcy-4 and gcy-22 confers iodide responsiveness on ASE-deficient animals. (A) Pansensory expression of gcy-4 rescues the gcy-4 mutant phenotype, as observed with the independent extrachromosomal arrays expressing osm-6prom::gcy-4 and one chromosomal integrant generated from one of the extrachromosomal arrays. (B) Pansensory expression of gcy-22 rescues the gcy-22 mutant phenotype, as observed with the independent extrachromosomal arrays expressing osm-6prom::gcy-22. Panel A and B are control experiments that establish the functionality of the individual transgenes. (C) A transgenic strain that expresses the integrated osm-6prom::gcy-4 array from A or one extrachromosomal osm-6prom::gcy-22 array is not able to rescue the loss of ASE neuron functionally (che-1 mutant background in which ASE fail to differentiate and do not express gcy-4 or gcy-22). However, combining the osm-6prom::gcy-4 integrated array with the osm-6prom::gcy-22 extrachromosomal array results in rescue of the che-1 mutant phenotype (last bar). (D) ASI-specific expression of GCY-4 and GCY-22 compensates for loss of che-1. Three lines that coexpress srg-47prom::gcy-4 and srg-47prom::gcy-22 arrays are able to rescue the chemotaxis defect of che-1 mutants to iodide. In all panels, error bars indicate the SEM. Analysis was completed using a one-way ANOVA with repeated measures comparing the mean of each group to the mean of the mutant. Error bars indicate SEM. The Holm\x{2013}Sidak correction was used to adjust for multiple comparisons and a = 0.01. All P-values reported are the adjusted value after the correction was applied. P-values: ***P < 0.001, **P < 0.01, *P < 0.05; NS, not significant (P < 0.05). In A\x{2013}C, n = 4 and in D, n = 3 with each sample being the average of the duplicate of two plates with four worms per plate. Assays were done blind to the genotype under test.