Abstract
Objective
To investigate the potential effects of eleven of the most commonly used Saudi folk herbal medications on the metabolic activity of CYP2C9 in human liver microsomes.
Method
CYP2C9-mediated 4′-hydroxylation of tolbutamide (TB) to 4′-hydroxytolbutamide (4-OH-TB) was utilized to assess the metabolic activity of CYP2C9. In the present study, an initial screening of the eleven herbs was carried out by incubating TB with microsomes and NADPH in absence or presence of a fixed concentration (25 μg/ml) of alcoholic extracts of different herbs and the metabolite formed was measured by HPLC. Herbs that showed significant effects were further investigated at a lower range of concentration.
Results
Among the investigated herbal extracts, only aniseed and curcuma showed statistically significant effects on the formation of 4-OH-TB in human liver microsomes. Curcuma produced a potent inhibition on the metabolite formation and its maximum (about 45% inhibition) was observed at the highest extract concentrations (10 and 25 μg/ml). On the other hand, aniseed significantly activates the formation of 4-OH-TB and the maximum activation (about 55%) was observed at 2.5 μg/ml of aniseed extract.
Conclusion
The results of this study have shown that alcoholic extracts of curcuma and aniseed were capable of inhibiting and activating; respectively, the CYP2C9-mediated 4-OH-TB formation in human liver microsomes, suggesting that these herbs have the potential to interact with CYP2C9 drug substrates. None of the other nine investigated herbs was able to produce any statistically significant effect.
Keywords: Herb, Interaction, CYP2C9, Tolbutamide, Metabolism, Human liver microsomes
1. Introduction
Use of herbal products has increased steadily among adults over the past few years. In fact, many people think that all herbs are safe owing to their natural origin. However, herbs may interact with conventional medications either taken as over the counter or prescribed by physicians resulting in various side effects (Klepser and Klepser, 1999; Izzo and Ernst, 2001). Such interactions may result in a new side effect that is not seen with the use of the herb or drug alone (Bailey and Dresser, 2004; Jankel and Fitterman, 1993). In United States of America, it was reported that about one third of people were using at least one type of alternative therapy (Eisenberg et al., 1993). In some other countries the proportions of patients who were using alternative medicine over one year were about 23% in Denmark, 49% in France, 48.5% in Australia and 24% in Saudi Arabia (Ioannides, 2002; Maclennan et al., 1996; Fisher and Ward, 1994; Al-Rowais, 2002).
A number of studies have reported many herbal product-drug interactions (Huang and Lesko, 2004). Some of the recent investigations have suggested the modulation of cytochrome P-450 enzymes (CYP)-mediated drug elimination as a major mechanism responsible for such types of interactions (Gurley et al., 2002; David et al., 2002). CYP2C9 has been considered as one of the most important drug-metabolizing enzymes in human judging by the number of substrates metabolized by this enzyme, which include nonsteroidal anti-inflammatory drugs, hypoglycemic agents and some narrow therapeutic index drugs such as (S)-warfarin and phenytoin. CYP2C9 has been shown to be polymorphic and different variants of the enzyme have been identified (Lee et al., 2002). Its genetic polymorphism is believed to contribute to the high inter-individual variability in the pharmacokinetics of some of its substrates.
Although there have been many reports on food-drug and herb-drug interactions by modulation of drug metabolism, most of these studies have focused on CYP3A4 enzyme (Diamond et al., 2000; Galluzzi et al., 2000; Guyonnet et al., 2000; Yang et al., 2001; Zhou et al., 2004; Hermann et al., 2002; Gross et al., 1999; Fuhr et al., 2002; Goho, 2001; Libersa et al., 2000; Lilja et al., 2000) and only few reports are available on the role of CYP2C9 in such interactions. One study has reported that pomegranate juice inhibits human CYP2C9 activity in vitro (Nagata et al., 2007). Recently, pine-apple juice was found to significantly inhibit CYP2C9 enzyme in human microsomes (Hidaka et al., 2008). Beside fruit juices, there are few reports wherein activity of CYP2C9 enzyme was modified by certain herbal constituents. For instance, polysaccharide peptides from COV-1 strain of Coriolus versicolor inhibited tolbutamide (TB) 4-hydroxylation in rats both in vitro and in vivo (Yeung et al., 2006). Ginkgo biloba extract was shown to modify hypoglycemic action of tolbutamide via competitive inhibition of (S)-warfarin 7-hydroxylase in rat liver microsomes (Sugiyama et al., 2004). Treatment with Angelica dahurica root extract inhibited the metabolism of tolbutamide, nifedipine and bufuralol in rats (Ishihara et al., 2000).
In addition to the limited availability of herb-drug interaction studies involving CYP2C9, there is a lack of information about the potential interactions between the commonly used herbal medications in Saudi Arabia and conventional drugs. One study has shown that the active ingredient of curcuma, cucumin, was able to inhibit the metabolic activities of several human recombinant CYP enzymes including CYP2C9 (Appiah-Opong et al., 2007). Therefore, the objective of this study was to investigate the potential effects of eleven of the most commonly used Saudi folk herbal products on the metabolic activity of CYP2C9 in human liver microsomes using TB as a probe substrate. The investigated herbs are boswellia, myrrh, asafoetida, curcuma, ginger, aloe, fenugreek, black seed, garden cress, cumin and aniseed.
2. Materials and methods
2.1. Materials
Human liver microsomes (protein concentration: 20 mg/ml) were purchased from Human Biologics International, Inc., Scottsdale, USA), shipped in small vials, on dry ice and stored at −80 °C. NADPH was purchased from Helix Pomatia, ICN Biomedicals Inc., Costa Mesa, AC, USA. Tolbutamide and 4-hydroytolbutamide (4-OH-TB) were purchased from Sigma–Aldrich, USA. Nitrazepam was of BP reference standard. All chemicals used were of the highest available commercial purity. All the herbs were purchased in dry form from Saudi market. General-purpose reagents (GPR) were used for extraction processes, while HPLC grade solvents were used for HPLC determinations. All other materials are of analytical grade.
2.2. Herb extraction
Each dried powdered herb (100 g) was exhaustively extracted by cold maceration with ethanol for 5 days; extract was filtered and concentrated at 40 °C under reduced pressure using a Buchi rotatory evaporator. The concentrations of the produced extracts to the weight of dried samples (% w/w) were found to be 34.8 for boswellia, 24 for myrrh, 31.6 for asafoetida, 5.1 for curcuma, 6.4 for ginger, 38.7 for aloe, 6.2 for fenugreek, 11.2 for black seed, 7.5 for garden cress, 6.4 for cumin and 5.0 for aniseed. The extracts were serially diluted using 96% ethanol to produce stock solutions, which were kept in a refrigerator until used.
2.3. Microsomal incubation
Herbal alcoholic extracts were transferred to clean tubes and alcohol was evaporated under nitrogen. Incubation conditions were selected based on previous studies (Ho and Moody, 1993; Miners et al., 1988) and preliminary experiments that involved the use of different incubation times and different microsomal protein concentrations. TB (5.0 μl of 15 mM to give a final concentration of 0.15 mM) was added to the tubes before the addition of human liver microsomes (0.25 mg protein/ml final concentration) and potassium phosphate buffer (0.1 M, pH 7.4), then mixed gently, and pre-incubated in a shaker water bath at 37 °C for 3.0 min. The reaction was initiated by the addition of 25 μl of 20 mM NADPH (1.0 mM final concentration) to complete a final volume of 0.5 ml, and incubated for a further 30 min. Control samples were treated the same way except that no herbal extracts were added. The reaction was terminated by the addition of cold methanol (250 μl) with vigorous shaking for 2.0 min. Nitrazepam (25 μl) from a stock solution of 1.0 μg/ml was added as an internal standard to each tube. The mixture was centrifuged at 12,000 rpm for 10 min; a 500 μl of the supernatant was transferred to HPLC autosampler vial and 50 μl of that was injected for analysis. Formation of the metabolite was found in the preliminary experiments to be linear with respect to time and protein concentration under the described conditions. Since it is impossible to predict the concentrations of the herbal constituents at the site of metabolism in vivo, the eleven herbs were initially screened at a relatively high fixed concentration (25 μg/ml) and then herbs that showed greater than 20% modulation were further investigated at a lower range of concentration.
2.4. Analysis of 4-hydroxytolbutamide (4-OH-TB) in human liver microsomes
4-OH-TB in human liver microsomes was analyzed using previously published HPLC method with slight modification (Miners et al., 1988). A symmetry column (150 × 3.9 mm ID, 5.0 μm) purchased from Waters®, Milford Massachusetts, USA, was used for this assay. Mobile phase consisted of acetonitrile: 0.05 M potassium dihydrogen orthophosphate (30:70; pH adjusted to 3.4 with orthophosphoric acid). The flow rate was 1 ml/min. The detector was operated at fixed wave length at 240 nm. Stock solutions of tolbutamide, and 4-OH-TB were prepared in acetonitrile and methanol; respectively. Calibration curve for tolbutamide metabolite was constructed using a concentration range of 0.05–1.0 μg/ml. Each calibration curve was prepared to contain a fixed concentration of tolbutamide (0.15 mM), microsomes and buffer but without the addition of NADPH.
2.5. Statistical analysis
Formation of 4-OH-TB in the presence of herbs was compared to that of control using one-way analysis of variance (ANOVA), and a post hoc Tukey’s multiple comparison test, with a P ⩽ 0.05 considered significant.
3. Results and discussion
In this in vitro microsomal study, boswellia, myrrh, asafoetida, curcuma, ginger, aloe, fenugreek, black seed, garden cress, cumin and aniseed were investigated for their effects on the metabolic activity of one of the major drug metabolizing enzyme, CYP2C9. Effects of the herbs were evaluated based on the formation of CYP2C9-mediated 4-OH-TB from tolbutamide in human liver microsomes.
Initially, a fixed concentration of the eleven herbs (25 μg/ml) was used to evaluate the potential effects on CYP2C9 (Fig. 1). Herbs that produced greater than 20% modulation in the formation of the metabolite were further evaluated at a lower concentration range of the herbs. Based on that, curcuma, ginger and aniseed were investigated at a concentration range of 1.0–25 μg/ml. Curcuma produced a potent inhibition on the metabolite formation and its maximum (about 45% inhibition) was observed at the highest extract concentrations (10 and 25 μg/ml) (Fig. 2). The maximum inhibition produced by ginger was about 25% but it was not statistically significant (Fig. 3). On the other hand, aniseed significantly activate the formation of 4-OH-TB and the maximum activation (about 55%) was observed at 2.5 μg/ml of aniseed extract as depicted in Fig. 4.
Figure 1.
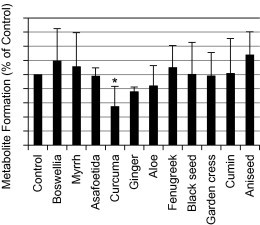
Effects of different herbs (at a fixed concentration) on the formation of 4-OH-TB from tolbutamide in human liver microsomes (mean + SD, *P < 0.05).
Figure 2.
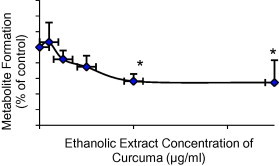
Effect of curcuma on the formation of 4-OH-TB from tolbutamide in human liver microsomes (mean + SD, *P < 0.05).
Figure 3.
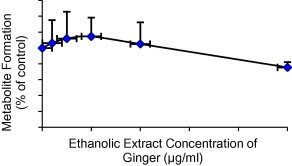
Effect of ginger on the formation of 4-OH-TB from tolbutamide in human liver microsomes (mean + SD).
Figure 4.
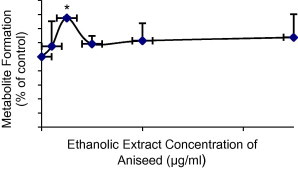
Effect of aniseed on the formation of 4-OH-TB from tolbutamide in human liver microsomes (mean + SD, *P < 0.05).
Although the use of alcoholic extracts provides a major advantage in screening herbal products for drug interactions, results obtained from such studies may not provide complete information about the mechanisms of interactions. A major reason for that is the presence of many herbal constituents in each extract, which make it difficult to relate an effect to certain compound(s). The observed inhibitory effect of CYP2C9 by curcuma is consistent with what has been reported by Appiah-Opong et al. (2007). However, the experimental model used in that study was based on recombinant CYP enzymes that were individually expressed in Escherichia coli, instead of human liver microsomes. They have shown significant inhibitory activities by the active ingredient of curcuma, cucumin, against five human CYP enzymes including CYP2C9, which suggests that the observed curcuma effect in this study is, at least partly, due to curcumin. The inhibition of CYP2C9 observed in their study suggests a non-competitive type of inhibition.
Activation of CYP2C9-mediated reactions has been reported in several previous studies (Korzekwa et al., 1998; Hutzler et al., 2001, 2002, 2003; Tracy, 2006; Atkins et al., 2001). However, activation of CYP2C9 by aniseed has not been shown before. A possible mechanism for the cause of CYP2C9 activation, which has been previously suggested by several investigators, is the presence of multiple binding sites in the enzyme active site (Korzekwa et al., 1998; Hutzler et al., 2001, 2002, 2003; Tracy, 2006; Atkins et al., 2001). This is supported by the fact that several CYP2C9 activators are known to be also substrates of the enzyme. Based on this hypothesis, the activation by aniseed observed in this study could be due to the ability of one (or more) of aniseed constituents to bind to a CYP2C9 binding site leading to an increase in the rate of tolbutamide metabolism possibly by inducing conformational changes in the enzyme. Constituents of aniseed include anethole, estragole, coumarins and flavonoids (Newall et al., 1996). Since CYP2C9 substrates include coumarin derivatives such as warfarin, some coumarin constituents in aniseed may have the structural requirements for binding to CYP2C9 and modulating its metabolic activity. Further in vitro studies that investigate the potential effects of individual aniseed constituents would be helpful in understanding the mechanism of CYP2C9 activation observed in this study.
It is concluded that among the eleven investigated herbs in this study, only curcuma and aniseed were capable of significantly modulating CYP2C9 metabolic activity. These two herbs therefore, have the potential to interact with conventional medicines eliminated from the body by CYP2C9. Some of these substrates such as S-warfarin and phenytoin are known to have narrow therapeutic margins and therefore, any alteration in their metabolism could result in serious clinical consequences even at their normal therapeutic doses (Gilbar and Brodribb, 2001; Murphy and Wilbur, 2003; Suvarna et al., 2003).
Acknowledgements
This study has been supported by the Deanship of Scientific Research (Grant number: DSR-AR-2-20) and the College of Pharmacy Research Center (Grant number CPRC-132), King Saud University, Riyadh, Saudi Arabia.
References
- Al-Rowais N.A. Herbal medicine in the treatment of diabetes mellitus. Saudi Med. J. 2002;23(11):1327–1331. [PubMed] [Google Scholar]
- Appiah-Opong R., Commandeur J.N., Vugt-Lussenburg B.V., Vermeulen N.P. Inhibition of human recombinant cytochrome P450s by curcumin and curcumin decomposition products. Toxicology. 2007;235:83–91. doi: 10.1016/j.tox.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Atkins W.M., Wang R.W., Lu A.H. Allosteric behavior in cytochrome P450-dependent in vitro drug-drug interactions: a prospective based on conformational dynamics. Chem. Res. Toxicol. 2001;14:338–347. doi: 10.1021/tx0002132. [DOI] [PubMed] [Google Scholar]
- Bailey D.G., Dresser G.K. Natural products and adverse drug interactions. CMAJ. 2004;170:1531–1532. doi: 10.1503/cmaj.1031558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David F.V., Sandeep M., Maurice D. Structure-activity relationship for human cytochrome P450 substrates and inhibitors. Drug Metab. Rev. 2002;34:69–82. doi: 10.1081/dmr-120001391. [DOI] [PubMed] [Google Scholar]
- Diamond B.J., Shifflett S.C., Feiwel N. Ginkgo biloba extract: mechanisms and clinical indications. Arch. Phys. Med. Rehabilat. 2000;81(5):668–678. doi: 10.1016/s0003-9993(00)90052-2. [DOI] [PubMed] [Google Scholar]
- Eisenberg D.M., Kessler R.C., Foster C., Norlock F.E., Calkins D.R., Delbanco T.L. Unconventional medicine in the United States: prevalence, cost and patterns of use. N. Engl. J. Med. 1993;328(4):246–252. doi: 10.1056/NEJM199301283280406. [DOI] [PubMed] [Google Scholar]
- Fisher P., Ward A. Complementary medicine in Europe. Br. Med. J. 1994;309(6947):107–111. doi: 10.1136/bmj.309.6947.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhr U., Muller-Peltzer H., Kern R., Lopez-Rojas P., Junemann M., Harder S., Staib A.H. Effects of grapefruit juice and smoking on verapamil concentrations in steady state. Eur. J. Clin. Pharmacol. 2002;58(1):45–53. doi: 10.1007/s00228-002-0436-7. [DOI] [PubMed] [Google Scholar]
- Galluzzi S., Zanetti O., Binetti G. Coma in a patient with Alzheimer’s disease taking low dose trazodone and ginkgo biloba. J. Neurol. Neuro. Surg. Psychiatr. 2000;68(5):679–683. doi: 10.1136/jnnp.68.5.679a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbar P.J., Brodribb T.R. Phenytoin and fluorouracil interaction. Ann. Pharmacother. 2001;35:1367–1370. doi: 10.1345/aph.1A051. [DOI] [PubMed] [Google Scholar]
- Goho C. Oral midazolam grapefruit juice drug interaction. Pediatr. Dent. 2001;23(4):365–366. [PubMed] [Google Scholar]
- Gross A.S., Goh Y.D., Addison R.S., Shenfield G.M. Influence of grapefruit juice on cisapride pharmacokinetics. Clin. Pharmacol. Ther. 1999;65(4):395–401. doi: 10.1016/S0009-9236(99)70133-5. [DOI] [PubMed] [Google Scholar]
- Gurley B.J., Gardner S.F., Hubbard M.A., Williams D.K., Gentry W.B., Ang Y.W. Cytochrome P450 phenotypic ratios for predicting herb-drug interactions in humans. Clin. Pharmacol. Ther. 2002;72(3):276–287. doi: 10.1067/mcp.2002.126913. [DOI] [PubMed] [Google Scholar]
- Guyonnet D., Belloir C., Suscetet M., Siess M.H., Le Bon A.M. Liver subcellular fractions from rats treated by organosulfur compounds from Allium modulate mutagen activation. Mutat. Res. 2000;466(1):17–26. doi: 10.1016/s1383-5718(99)00234-x. [DOI] [PubMed] [Google Scholar]
- Hermann M., Asberg A., Reubsaet J.L., Sather S., Berg K.J., Christensen H. Intake of grapefruit juice alters the metabolic pattern of cyclosporine in renal transplant recipients. Int. Clin. Pharmacol. Ther. 2002;40(10):451–456. doi: 10.5414/cpp40451. [DOI] [PubMed] [Google Scholar]
- Hidaka M., Nagata M., Kawano Y., Sekiya H., Kai H., Yamasaki K., Okumura M., Arimori K. Inhibitory effects of fruit juices on cytochrome P450 2C9 activity in vitro. Biosci. Biotechnol. Biochem. 2008;72(2):406–411. doi: 10.1271/bbb.70511. [DOI] [PubMed] [Google Scholar]
- Ho J.W., Moody D.E. Determination of tolbutamide hydroxylation in rat liver microsomes by high-performance liquid chromatography: effect of psychoactive drugs on in vitro activity. Life Sci. 1993;52(1):21–28. doi: 10.1016/0024-3205(93)90284-a. [DOI] [PubMed] [Google Scholar]
- Huang S.M., Lesko L.J. Drug–drug, drug-dietary supplement, and drug-citrus fruit and other food interactions: what have we learned? J. Clin. Pharmacol. 2004;44(6):559–569. doi: 10.1177/0091270004265367. [DOI] [PubMed] [Google Scholar]
- Hutzler J.M., Hauer M.J., Tracy T.S. Dapsone activation of CYP2C9-mediated metabolism: evidence for activation of multiple substrates and a two-site model. Drug Metab. Dispos. 2001;29:1–6. [PubMed] [Google Scholar]
- Hutzler J.M., Kolwankar D., Hummel M.A., Tracy T.S. Activation of CYP2C9-mediated metabolism by a series of dapsone analogs: kinetics and structural requirements. Drug Metab. Dispos. 2002;30(11):1194–1200. doi: 10.1124/dmd.30.11.1194. [DOI] [PubMed] [Google Scholar]
- Hutzler J.M., Wienkers L.C., Wahlstrom J.L., Carlson T.J., Tracy T.S., Hutzler J.M., Wienkers L.C., Wahlstrom J.L., Carlson T.J., Tracy T.S. Activation of cytochrome P450 2C9-mediated metabolism: mechanistic evidence in support of kinetic observations. Arch. Biochem. Biophys. 2003;410(1):16–24. doi: 10.1016/s0003-9861(02)00665-3. [DOI] [PubMed] [Google Scholar]
- Ioannides C. Pharmacokinetic interactions between herbal remedies and medicinal drug. Xenobiotica. 2002;32(6):451–478. doi: 10.1080/00498250210124147. [DOI] [PubMed] [Google Scholar]
- Ishihara K., Kushida H., Yuzurihara M., Wakui Y., Yanagisawa T., Kamei H., Ohmori S., Kitada M. Interaction of drugs and Chinese herbs: pharmacokinetic changes of tolbutamide and diazepam caused by extract of Angelica dahurica. J. Pharm. Pharmacol. 2000;52(8):1023–1029. doi: 10.1211/0022357001774750. [DOI] [PubMed] [Google Scholar]
- Izzo A.A., Ernst E. Interactions between herbal medicine-sand prescribed drug: a systematic review. Drugs. 2001;61(15):2163–2175. doi: 10.2165/00003495-200161150-00002. [DOI] [PubMed] [Google Scholar]
- Jankel C.A., Fitterman L.K. Epidemiology of drug–drug interactions as a cause of hospital admissions. Drug Safety. 1993;9(1):51–59. doi: 10.2165/00002018-199309010-00005. [DOI] [PubMed] [Google Scholar]
- Klepser T.B., Klepser M.E. Unsafe and potentially safe herbal therapies. Am. J. Health Syst. Pharm. 1999;56(2):125–138. doi: 10.1093/ajhp/56.2.125. [DOI] [PubMed] [Google Scholar]
- Korzekwa K.R., Krishnamachary N., Shou M., Ogai A., Parise R.A., Rettie A.E., Gonzalez F.J., Tracy T.S. Evaluation of atypical cytochrome P450 kinetics with two-substrate models: evidence that multiple substrates can simultaneously bind to cytochrome P450 active sites. Biochemistry. 1998;37(12):4137–4147. doi: 10.1021/bi9715627. [DOI] [PubMed] [Google Scholar]
- Lee C.R., Goldstein J.A., Pieper J.A. Cytochrome P4502C9 polymorphisms: a comprehensive review of the in vitro and human data. Pharmacogenetics. 2002;12:251–263. doi: 10.1097/00008571-200204000-00010. [DOI] [PubMed] [Google Scholar]
- Libersa C.C., Brique S.A., Motte K.B., Caron J.F., Guedon-Moreau L.M., Humber L., Vincent A., Devos P., Lhermitte M.A. Dramatic inhibition of amiodarone metabolism induced by grapefruit juice. Br. J. Clin. Pharmacol. 2000;49(4):373–378. doi: 10.1046/j.1365-2125.2000.00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilja J.J., Kivisto K.T., Neuvonen P.J. Duration of effect of grapefruit juice on the pharmacokinetics of the CYP3A4 substrate simvastatin. Clin. Pharmacol. Ther. 2000;68(4):384–390. doi: 10.1067/mcp.2000.110216. [DOI] [PubMed] [Google Scholar]
- Maclennan A.H., Wilson D.H., Taylor A.W. Prevalence and cost of alternative medicine in Australia. Lancet. 1996;347(9001):569–573. doi: 10.1016/s0140-6736(96)91271-4. [DOI] [PubMed] [Google Scholar]
- Miners J.O., Smith K.J., Robson R.A., McManus M.E., Veronese M.E., Birkett D.J. Tolbutamide hydroxylation by human liver microsomes: kinetic characterization and relationship to other cytochrome P-450 dependent xenobiotic oxidations. Biochem. Pharmacol. 1988;37:1137–1144. doi: 10.1016/0006-2952(88)90522-9. [DOI] [PubMed] [Google Scholar]
- Murphy A., Wilbur K. Phenytoin–diazepam interaction. Ann. Pharmacother. 2003;37:659–663. doi: 10.1345/aph.1C413. [DOI] [PubMed] [Google Scholar]
- Nagata M., Hidaka M., Sekiya H., Kawano Y., Yamasaki K., Okumura M., Arimori K. Effects of pomegranate juice on human cytochrome P450 2C9 and tolbutamide pharmacokinetics in rats. Drug Metab. Dispos. 2007;35(2):302–305. doi: 10.1124/dmd.106.011718. [DOI] [PubMed] [Google Scholar]
- Newall C.A., Anderson L.A., Phillipson J.D. Pharmaceutical Press; London, England: 1996. Herbal Medicines. pp. 30–31. [Google Scholar]
- Sugiyama T., Kubota Y., Shinozuka K., Yamada S., Wu J., Umegaki K. Ginkgo biloba extract modifies hypoglycemic action of tolbutamide via hepatic cytochrome P450 mediated mechanism in aged rats. Life Sci. 2004;75(9):1113–1122. doi: 10.1016/j.lfs.2004.02.020. [DOI] [PubMed] [Google Scholar]
- Suvarna R., Pirmohamed M., Henderson L. Possible interaction between warfarin and cranberry juice. BMJ. 2003;327:1454. doi: 10.1136/bmj.327.7429.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy T.S. Atypical cytochrome p450 kinetics: implications for drug discovery. Drugs R.D. 2006;7:349–363. doi: 10.2165/00126839-200607060-00004. [DOI] [PubMed] [Google Scholar]
- Yang C.S., Chhabra S.K., Hong J.Y., Smith T.J. Mechanisms of inhibition of chemical toxicity and carcinogenesis by diallyl sulfide (DAS) and related compound. J. Nutr. 2001;131(3s):1041S–1045S. doi: 10.1093/jn/131.3.1041S. [DOI] [PubMed] [Google Scholar]
- Yeung J.H., Chan S.L., Or P.M. Polysaccharide peptides from COV-1 strain of Coriolus versicolor inhibit tolbutamide 4-hydroxylation in the rat in vitro and in vivo. Food Chem. Toxicol. 2006;44(8):1414–1423. doi: 10.1016/j.fct.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Zhou S., Chan E., Pan S.Q., Huang M., Lee E.J. Pharmacokinetic interactions of drugs with St. John’s Wort. J. Psychpharmacol. 2004;18(2):262–276. doi: 10.1177/0269881104042632. [DOI] [PubMed] [Google Scholar]


