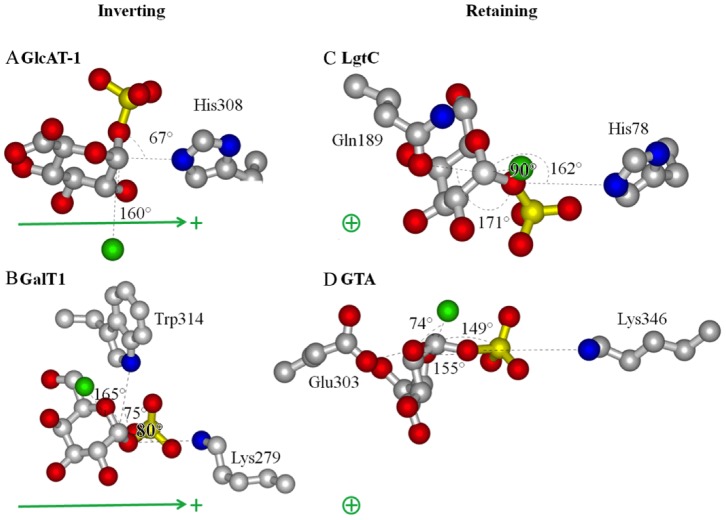
Figure 3. Reaction center dipoles.
Opposed to the placement of the acceptor nucleophile (Green spheres), the closest polar residues to leaving group β-phosphate O3 and C1 lay acutely (67° and 75°, respectively) for inverting enzymes (A,B) and lie nearly in-line (171° and 155°, respectively) for retaining enzymes GTA (C,D). This may help to stabilize the associative intermediates without hindering the opposite angle of attack from the acceptor molecule nucleophile. Also, the O3– C1vectors lay looselyperpendicular to the enzyme macrodipole vectors to stabilize the inverting transition states (green arrows) (A,B), and loosely parallel to stabilize the retaining transition states (C,D) (green ⊕, dipole oriented with the cationic end above the page and the anionic end in the page).