Abstract
Background:
Ferumoxytol is a safe and effective parenteral therapy used for the treatment of iron deficiency anaemia that has recently been approved for use in North America and in Europe.
Methods:
Ferumoxytol consists of a superparamagnetic iron oxide (SPIO) core, which causes T1, T2 and T2* shortening effects, and a carbohydrate shell, which results in a prolonged intravascular half life.
Results:
These properties are under-reported and not well recognised. They can interfere with MRI interpretation, potentially masking enhancement and rendering examinations non-diagnostic or simulating pathologic disease states. Both radiologists and non-radiologist physicians must consider the potential interaction of ferumoxytol with MRI when interpreting and prescribing MRI examinations in their patients.
Main Messages
• Ferumoxytol has recently been approved for the treatment of iron deficiency anaemia.
• Ferumoxytol is a small iron oxide particle with prolonged intravascular half life and T1, T2 and T2* shortening effects.
• Administration of ferumoxytol can mask enhancement, rendering MRI studies potentially non-diagnostic.
• Ferumoxytol can mimic diseases such as haemosiderosis, haemochromatosis and superficial siderosis.
• Ferumoxytol interactions with MRI must be recognised by radiologists and non-radiologist physicians.
Keywords: Ferumoxytol, Gadolinium, Magnetic resonance imaging, Small iron oxide particle, Iron
Case presentation
A 54-year-old male patient with nonalcoholic steatohepatitis (NASH) cirrhosis presented for surveillance with magnetic resonance imaging (MRI) of the liver to screen for hepatocellular carcinoma in the setting of a routine pre-transplant workup. The patient had iron deficiency anaemia and received parenteral ferumoxytol (second intravenous dose of 510 mg) 3 days prior to the MRI examination. MRI was performed using our institution’s standard high-risk liver protocol on a 3.0-T MRI scanner (Discovery 750w, General Electric Medical Systems, Milwaukee, WI). Pulse sequences included: axial and coronal T2-weighted single-shot fast spin echo (ssFSE), axial T2-weighted fast recovery fast spin echo (FRFSE), axial 3D in- and out-of-phase gradient recalled echo (GRE), B50 and B500 diffusion-weighted imaging with an apparent diffusion coefficient map, and pre- and dynamic post-contrast fat-suppressed 3D T1-weighted GRE sequences (LAVA), including arterial, portal venous and equilibrium phases. Gadobutrol (Gadovist, Bayer Inc., Toronto, ON) was administered to obtain the post-contrast imaging. The MRI study showed interval changes from the baseline examination performed 15 months prior. In the interval there was a marked reduction in signal intensity of the liver and spleen on T2 ssFSE sequences suggesting iron overload, which had not been present on the previous examination (Fig. 1). Portahepatic lymph nodes also demonstrated low T2 signal intensity (Fig. 1). Imaging findings of iron deposition in the bone marrow were revealed on T1 in-phase GRE imaging and also had not been present on the previous examination (Fig. 2). Routine pre-contrast T1 GRE images demonstrated increased signal intensity in the blood pool and abdominal organs, which masked normal enhancement after gadolinium was administered (Fig. 2). The constellation of findings was based on T1 and T2 shortening effects from ferumoxytol, which rendered the study non-diagnostic. The patient also had recently undergone unenhanced MRI of the brain to evaluate the progressive cognitive decline 1 day before the abdominal MRI, which demonstrated diffuse low signal intensity along the surface of the brain on T2* GRE images, findings also related to ferumoxytol administration that mimic superficial siderosis (Fig. 3). The patient provided informed consent for publication.
Fig. 1.
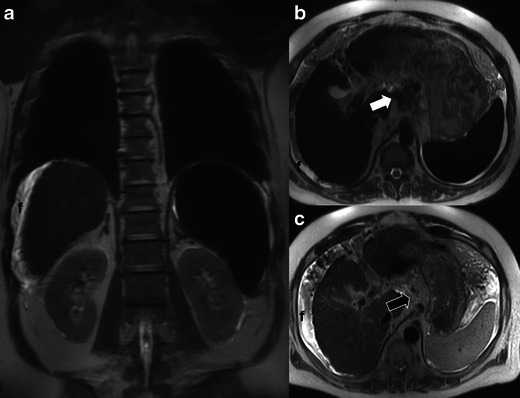
Coronal (a) and axial (b) T2 single shot fast spin echo (ssFSE) images (TE 90/TR 1400) demonstrate marked reduction in signal intensity of the liver and spleen. Portahepatic lymph nodes (white arrow in b) also demonstrate low T2 signal intensity. Axial T2 ssFSE image from baseline MRI performed 15 months prior (c) demonstrates normal signal intensity of the liver, spleen and portal lymph nodes (black arrow in c), which are barely perceptible on this non-fat suppressed image. Low signal intensity in the liver, spleen and lymph nodes in a and b is due to ferumoxytol, which was administered just prior to the examination. Also note the morphologic features of cirrhosis, splenomegaly and a small volume of peri-hepatic fluid
Fig. 2.
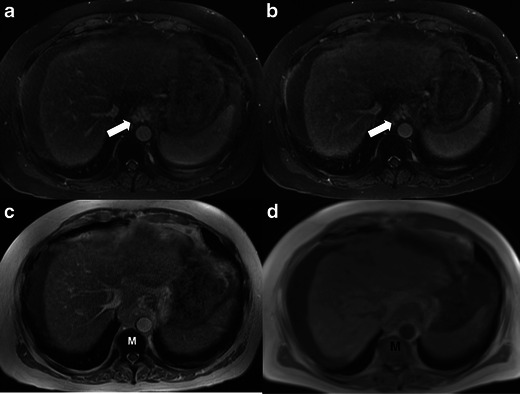
Axial pre- (a) and post-gadolinium (b) fat-suppressed T1 gradient recalled echo (GRE) images (1.7/4.3) show no appreciable change in the signal intensity of the blood pool or abdominal organs (liver, spleen) after gadolinium injection (b) due to the pre-existing T1 shortening effects of ferumoxytol, which masks the T1 shortening effects of gadolinium. This interaction renders the post-gadolinium images non-diagnostic. Note enhancement of gastroesophageal varices on both pre- and post-contrast images (white arrows). Axial T1 in-phase GRE image (2.3/4.3) performed prior to gadolinium injection (c) also depicts T1 shortening effects in the liver and spleen (which masks expected profound hypointensity based on T2 signal) and blood pool. Note the marked hypointensity of the bone marrow (M) in (c) due to marrow uptake of ferumoxytol when compared to the baseline axial T1 GRE (d) image performed 15 months earlier, which demonstrates normal marrow (M) signal intensity
Fig. 3.
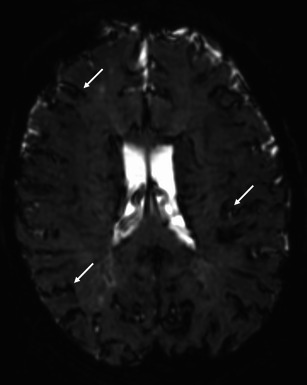
Axial T2* GRE image (40/3000) demonstrates low signal intensity along the surface of the brain (white arrows) presumably related to ferumoxytol accumulation within the leptomeninges. The appearance could easily be misinterpreted as superficial siderosis the history of ferumoxytol administration is not known
Discussion
Ferumoxytol (marketed in Europe as Rienso and North America as Feraheme by Takeda Inc. and AMAG Pharmaceuticals) has recently been approved in Europe and North America for the parenteral treatment of iron deficiency anaemia in patients with chronic kidney disease [1–3].
Parenteral iron therapy can be used safely to treat iron deficiency anaemia in patients with chronic kidney disease and other chronic diseases and represents an attractive alternative to oral iron therapy with more effective results based on a recent systematic review [4]. Feraheme or Rienso (30 mg/ml, ferumoxytol) is to be administered as an undiluted intravenous (IV) injection delivered at a rate of up to 1 ml/s (30 mg/s) for a total dose of 510 mg IV followed by a second 510-mg IV injection 2 to 8 days later [1]. Improved outcomes when compared to oral therapy [4] and intravenous bolus injection compared to other parenteral iron suspensions that require longer infusions will likely lead to increased use of ferumoxytol in clinical practice [5].
Ferumoxytol is a haematinic agent that consists of an ultrasmall superparamagnetic iron oxide (SPIO) core coated with a carbohydrate shell to help isolate the bioactive iron from plasma elements until it is taken up by the reticuloendothelial system (RES) macrophages of the liver, spleen and bone marrow [1]. Ferumoxytol, similar to other SPIO agents, results in T1, T2 and T2* shortening effects with MRI, which can impact image interpretation and clinical practice [5]. The pharmaceutical composition of ferumoxytol (SPIO encased in carbohydrate shell) results in a prolonged circulating half-life (14–15 h) in the intravascular space (blood pool), which can alter the relaxivity characteristics of the blood pool for days to months [5]. The T1 shortening effects and extremely long intravascular half-life of ferumoxytol render it particularly problematic with the use of traditional gadolinium-enhanced GRE pulse sequences. Extracellular gadolinium agents also rely on T1 shortening effects, which can be masked after patients have received ferumoxytol, potentially rendering examinations non-diagnostic. This effect has been recently reported [5] and occurred in our case. This effect could have further implications in other areas of MRI requiring the use of gadolinium for diagnosis including breast and cardiac imaging. Furthermore, failure to appreciate that ferumoxytol has been administered could result in the erroneous diagnosis of iron overload (haemosiderosis or haemochromatosis) in the abdomen. In our patient, superficial siderosis in the brain could have been erroneously diagnosed on GRE images of the brain presumably related to ferumoxytol accumulation in the leptomeninges. A key to diagnosis if the history of the ferumoxytol injection is not known or not provided is findings of enhancement of the blood pool on pre-contrast GRE sequences. Low signal intensity of lymph nodes on both T1 GRE and T2 pulse sequences is also an indicator that the patient may have received ferumoxytol, although this could also be seen with calcification.
The T1 shortening properties and prolonged intravascular half-life of ferumoxytol could represent an alternative to enhanced MRI in patients with compromised renal function who are at risk for nephrogenic systemic fibrosis when using traditional gadolinium contrast agents that are cleared by the kidneys [6]. Ferumoxytol, like other SPIO agents, is not cleared by glomerular filtration but by macrophages in the RES, and this feature could also serve to help detect pathology in the RES where normal uptake is not observed, such as in the diagnosis of metastatic lymphadenopathy [7].
The ferumoxytol package insert warns of potential MRI effects lasting for up to 3 months [5]. A recent prospective study that monitored iron clearance from the liver in patients receiving ferumoxytol showed a wide variability in iron clearance times ranging from 3 months to 11 months, with several participants showing persistent T2 shortening effects after 11 months [7].
Based on our local experience and the limited information available in the literature, our institution has developed guidelines for radiologists and non-radiologist physicians to consider when ordering, prescribing or interpreting MRI studies in patients receiving ferumoxytol. For patients who require MRI with anticipated treatment with ferumoxytol, it is suggested that the patient undergo MRI prior to initiation of therapy. For patients who have recently received the medication and require gadolinium-enhanced MRI, it is suggested that the exam be deferred for 3 months or that another imaging modality (such as computed tomography) be considered as an alternative where appropriate. For patients who have recently received ferumoxytol and require MRI without gadolinium, each case should be considered individually with anticipated problems likely occurring mostly in those pathologies that involve organs of the RES.
The potential interaction of ferumoxytol with clinical MRI studies can impact interpretation and is important for both radiologists and non-radiologist physicians to appreciate when requesting and interpreting MRI examinations for their patients. The recent administration of ferumoxytol can mask enhancement and render MRI examinations non-diagnostic. Furthermore, ferumoxytol can mimic disease states such as haemosiderosis or superficial siderosis. This interaction is under-reported and poorly understood by radiologists and the medical community at large. Increased use of ferumoxytol in patients with chronic illness and iron deficiency is expected and will result in a greater occurrence of potential interactions with MRIs.
Acknowledgments
Conflict of interest
The authors declare no conflicts of interest. No funding was received for this work.
Abbreviations
- SPIO
Super-paramagnetic iron oxide particle
- RES
Reticulo-endothelial cell system
- GRE
Gradient recalled echo
- FSE
Fast spin echo
References
- 1.Health Canada (2012) Summary basis of decision (SBD) for FERAHEME. Health Canada, Ottawa. Available via: http://www.hc-sc.gc.ca/dhp-mps/prodpharma/sbd-smd/drug-med/sbd_smd_2012_feraheme_133250-eng.php. Accessed 26 June 2012
- 2.Lu M, Cohen MH, Rieves D, Pazdur R. FDA report: ferumoxytol for intravenous iron therapy in adult patients with chronic kidney disease. Am J Hematol. 2010;85:315–319. doi: 10.1002/ajh.21656. [DOI] [PubMed] [Google Scholar]
- 3.European Medicines Agency (2012) Rienso: CHMP summary of positive opinion for Rienso. European Medicines Agency, London. Available via: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002215/human_med_001569.jsp&mid=WC0b01ac058001d124. Accessed 20 April 2012
- 4.Albaramki J, Hodson EM, Craig JC, Webster AC. Parenteral versus oral iron therapy for adults and children with chronic kidney disease. Cochrane Database Syst Rev. 2012;1:CD007857. doi: 10.1002/14651858.CD007857.pub2. [DOI] [PubMed] [Google Scholar]
- 5.McCullough BJ, Kolokythas O, Maki JH, Green DE. Ferumoxytol in clinical practice: implications for MRI. J Magn Reson Imaging. 2012;36(4):1–4. doi: 10.1002/jmri.23879. [DOI] [PubMed] [Google Scholar]
- 6.Neuwelt EA, Hamilton BE, Varallyay CG, Rooney WR, Edelmen RD, Jacobs PM, Watnick SG. Ultrasmall superparamagnetic iron oxides (USPIOs): a future alternative magnetic resonance (MR) contrast agent for patients at risk for nephrogenic systemic fibrosis? Kidney Int. 2009;75(5):465–474. doi: 10.1038/ki.2008.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Storey P, Lim RP, Chandarana H, Rosenkrantz AB, Kim D, Stoffel DR, Lee VS. MRI assessment of hepatic iron clearance rates after USPIO administration in healthy adults. Invest Radiol. 2012;47(12):717–724. doi: 10.1097/RLI.0b013e31826dc151. [DOI] [PubMed] [Google Scholar]


