Abstract
INTRODUCTION
Visually isoattenuating pancreatic adenocarcinoma is defined as a mass not directly visible on CT and recognizable only by secondary imaging signs. The frequency of isoattenuating pancreatic adenocarcinomas at dynamic-enhanced CT has been reported to range from 5.4% to 14%. Furthermore, 80% of the visually isoattenuating pancreatic adenocarcinomas are detectable in dynamic-enhanced MRI. Consequently, a pancreatic adenocarcinoma undetected in both the above imaging studies is an exceptionally rare event.
PRESENTATION OF CASE
The present report describes a case of a histologically proved 3.5 cm pancreatic adenocarcinoma undetected in both dynamic-enhanced CT and MRI. The patient presented with progressive jaundice over the preceding 20 days. Initial abdominal CT showed a dilated pancreatic and common bile duct without demonstration of a lesion responsible for the clinical and imaging findings. Additional diagnostic work-up with dynamic CT and dynamic MRI failed to reveal a definitive mass. ERCP revealed an irregular interruption of the pancreatic and distal common bile duct with upstream dilation. Blind radical pancreaticoduodenectomy was performed. Histologic examination showed a pT3pN1MO pancreatic ductal adenocarcinoma of the head/neck.
DISCUSSION
Isoattenuating pancreatic adenocarcinoma patients represent a small but meaningful subset of patients with pancreatic cancer, as they have better survival. The more favorable postsurgical survival makes it even more imperative to correctly diagnose their cases at early stages by obtaining further diagnostic work-up with dynamic pancreatic CT, dynamic MRI and endoscopic ultrasound.
CONCLUSION
When the above studies fail to unmask the lesion, blind pancreaticoduodenectomy should be based on strong clinical suspicion and secondary imaging findings.
Keywords: Isoattenuating pancreatic mass, Dynamic CT, Dynamic MRI, Endoscopic ultrasound, Blind pancreaticoduodenectomy
1. Introduction
Multidetector row and dynamic dual-phase scanning have substantially improved the accuracy of CT for detection of pancreatic cancer. However, some lesions, in which the tumor attenuation is indistinguishable from the attenuation of the pancreatic parenchyma, can still escape detection.1 The frequency of isoattenuating pancreatic adenocarcinomas at dynamic-enhanced CT among pathologically proved pancreatic cancers has been reported to range from 5.4% to 14%.2 Furthermore, 80% of the visually isoattenuating pancreatic adenocarcinomas at CT are detectable in dynamic-enhanced MRI.3 Consequently, a pancreatic adenocarcinoma undetected in both dynamic CT and MRI is an exceptionally rare event. Awareness and knowledge about this uncommon finding is important, as it could cause a missed or delayed diagnosis. The present report describes a case of a histologically proved isoattenuating pancreatic adenocarcinoma undetected in both dynamic-enhanced CT and MRI.
2. Presentation of case
A 62-year-old male patient referred to our surgical department owing to progressive jaundice associated with darkening of the urine and pruritus over the preceding 20 days. Direct questioning revealed a history of vague abdominal pain, anorexia and weight loss of approximately 6 kg over the last 3 months, symptoms which were attributed by the patient to exacerbation of peptic ulcer disease. There was no other previous medical history. The patient was a smoker (15–20 cigarettes per day for the last 35 years) and an occasional alcohol drinker.
At initial presentation jaundice, palpable gallbladder and muscle wasting were present on physical examination. Laboratory studies revealed a significant increase in serum total bilirubin (12.10 mg/dl), alkaline phosphatase (271 U/l) and γ-glutamyl transferase (85 U/l). Serum amylase (40 U/l), IgG (982 mg/dl) and IgG4 (56 mg/dl) levels were normal. Regarding tumor markers, level of CA 19-9 (60 U/ml) was elevated.
Transabdominal ultrasonography showed a dilated pancreatic duct (5 mm), dilated intrahepatic and extrahepatic bile ducts (common hepatic and bile duct ranged between 12.2 and 14.3 mm in diameter) without gallstones and focal hepatic or pancreatic lesions. Initial abdominal CT demonstrated interruption of pancreatic duct in the head/neck of pancreas with upstream pancreatic ductal dilation and biliary dilation, without any visible mass or nodule. Diagnostic ERCP also performed and depicted an irregular interruption of the pancreatic duct and narrowing in the distal common bile duct with upstream dilation; a plastic stent was placed across the biliary obstruction. Strong clinical suspicion and secondary imaging signs for pancreatic head cancer imposed additional diagnostic work-up. A dynamic-enhanced pancreatic CT examination obtained with a 16-multidetector row scanner according to a dual-phase pancreatic protocol in order to depict a definitive mass. However, no pancreatic lesion of increased or decreased attenuation compared with the normal pancreatic parenchyma was observed in both arterial and portal phases (Fig. 1). Furthermore, gadolinium-enhanced dynamic MRI examination with nonenhanced fat-saturated T1-/T2-weighted and contrast-enhanced arterial, venous and delayed phase fat-saturated T1-weighted images obtained, in order to expose the mass. Neither of these sequences achieved to demonstrate the lesion (Fig. 2). No evidence of metastatic disease and invasion into local structures was depicted.
Fig. 1.
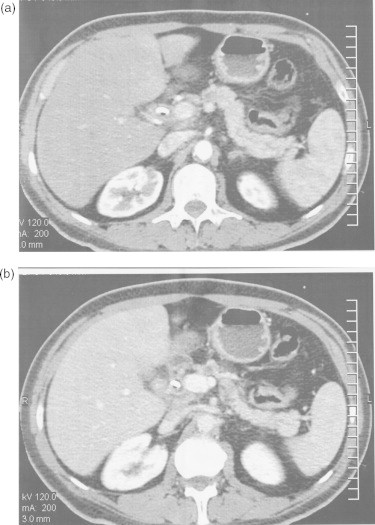
Transverse contrast-enhanced (a) arterial and (b) portal phase CT images demonstrated interruption of the pancreatic duct in the neck portion, as well as upstream pancreatic duct dilatation. Images did not provide visualization of mass.
Fig. 2.
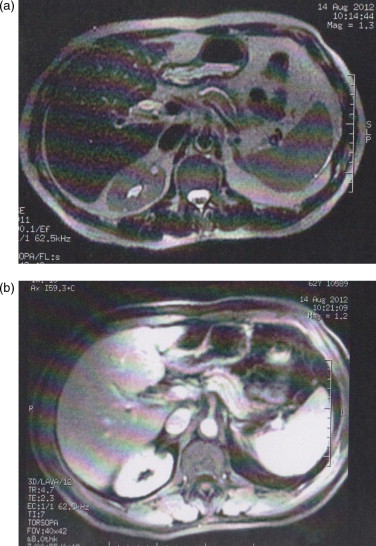
Transverse (a) nonenhanced T2-weighted and (b) arterial phase contrast-enhanced T1-weighted MR images failed to expose the mass.
Although preoperative imaging did not reveal a definitive mass, resection of the presumed pancreatic carcinoma was decided 9 days after presentation. The decision/making flow chart of the study patient is presented in Table 1.13 Radical pancreaticoduodenectomy was performed (standard resection plus distal gastrectomy and retroperitoneal lymph node dissection extending from the right renal hilum to the left lateral border of the aorta and from the portal vein to the inferior mesenteric artery) with Roux-en-Y reconstruction. In the surgical specimen the suspected mass appeared inconspicuous and permeated into the pancreatic parenchyma with indistinct margins and was recognizable only by its very hard consistency on palpation at the neck of the pancreas. Histologic examination showed a moderate differentiated, pT3pN1MO and stage IIB according to the AJCC cancer staging manual, negative margin pancreatic ductal adenocarcinoma of the head/neck. Postoperative course of the patient was uneventful and adjuvant interferon-based chemoradiation was performed.
Table 1.
Decision/making flow chart of the study patient.
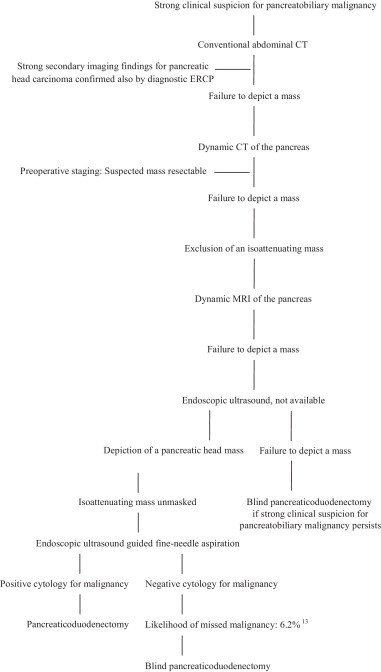
3. Discussion
Visually isoattenuating pancreatic adenocarcinoma is defined as a mass not directly visible on CT and recognizable only by secondary imaging signs, when both of the following criteria are fulfilled: (a) no pancreatic lesion of increased or decreased attenuation, compared with the pancreatic parenchyma is observed in both arterial and portal phases; (b) no CT findings of advanced chronic pancreatitis or severe obstructive pancreatitis are observed; and (c) secondary imaging findings such as interruption or obstruction of the pancreatic duct or pancreatic parenchymal atrophy, narrowing in the distal common bile duct, mass effect and/or convex contour abnormality are present.4
As mentioned above, isoattenuating pancreatic adenocarcinoma patients represent a small but meaningful subset of patients with pancreatic cancer. Kim et al., in one of the limited number of studies that have been referred in the literature regarding isoattenuating pancreatic adenocarcinoma patients, showed that these patients have a better survival than usual pancreatic adenocarcinoma patients. In their study, the median survival after curative-intent surgery was significantly longer in visually isoattenuating pancreatic adenocarcinoma patients (30 months vs. 15.6 months, p = 0.002). The adjusted hazard ratio for visually isoattenuating to usual pancreatic adenocarcinoma was 0.430, indicating that visually isoattenuating pancreatic adenocarcinoma was independently associated with a 57% reduced risk of death after curative-intent surgery.5 The more favorable postsurgical survival of these patients makes it even more imperative to correctly diagnose their cases at a stage when surgical resection is possible, by performing a thorough diagnostic work-up.
The reported sensitivity of dynamic multidetector-row CT (MDCT) in revealing pancreatic carcinoma is high, ranging between 89% and 97%.6 It should be taken into consideration when a suspected pancreatic adenocarcinoma is depicted as isoattenuating on conventional transverse CT.7 However, considerable limitations exist, like in our patient's case, as the frequency of visually isoattenuating pancreatic adenocarcinomas on dynamic-enhanced MDCT among pathologically proved pancreatic cancers has been reported to range from 5.4% to 14%. Gadolinium-enhanced dynamic MRI has been reported to be superior to MDCT for depicting: (a) small lesions; (b) local tumor extent and vascular involvement except for duodenal invasion and portal venous system involvement; and (c) isoattenuating masses, as can aid visualization of approximately 80% of visually isoattenuating pancreatic adenocarcinomas at CT.8 In our case both dynamic-enhanced CT and MRI failed to unmask the mass, which is an extremely rare event. Unfortunately in our patient's case endoscopic ultrasound (EUS) was not available; EUS would have offered us an excellent chance to describe the sonographic characteristics of an isoattenuating mass, as studies investigating the utility of EUS for detection of visually isoattenuating pancreatic adenocarcinomas could not be recovered in the literature and may be worthwhile. Moreover, EUS, if succeeded to unmask the mass, could have offered us the ability of EUS-guided fine needle aspiration (FNA) cytology.9 Although a negative result of EUS-FNA would not have modified surgical management, a positive diagnosis would definitely have supported us to perform a pancreaticoduodenectomy earlier, on the basis of high specificity and a positive predictive value.
When the above imaging studies fail to unmask the mass, many specialists justify blind pancreaticoduodenectomy (PD) for pancreatic head neoplasms with suspected but unproven malignancy, because: (a) preoperative diagnostic procedures may complicate the management or delay surgery, and delaying surgery may increase the likelihood that a tumor is unresectable or has metastasized; (b) a negative biopsy does not rule out cancer, and biopsy information does not affect the choice of therapy since a negative biopsy still commits the patients to surgery; and (c) there is a low incidence of benign diagnoses after blind PD.10 Indeed, Camp et al. studied 68 patients who underwent blind PD and found that 61 patients (90%) had a malignant and 7 (10%) had a benign histologically proved diagnosis of chronic inflammation/pancreatitis. The author concluded that blind PD should be based on combined clinical, CT and/or ERCP data, such in our case.11 In order to avoid a benign PD, Garcea et al. tried to evaluate the effectiveness of intraoperative confirmation of malignancy. In their study, 62 patients underwent intraoperative frozen section histology; 6.7% of them had a missed malignancy, but intraoperative histology prevented PD in 35% of patients with benign disease. However the author concluded that the chance of missing a small tumor with a false-negative biopsy will be unacceptable and he would prefer to undertake a “blind” resection and accept the mortality risk of PD for benign disease.12
4. Conclusion
Pancreatic adenocarcinomas, when isoattenuating, are not directly visible on CT. In such case, further diagnostic work-up with dynamic-enhanced pancreatic MDCT, MRI, and EUS should be obtained, when available, in order to depict a definitive mass. When the above studies fail to unmask the lesion, such in our patient case, blind PD should be based on strong clinical suspicion and secondary imaging studies.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Funding
The authors declare that they have no source of funding for this publication.
Ethical approval
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Author contributions
Konstantinos Boulas and Dimitrios Tselios equally contributed to the writing of this paper. Konstantinos Blouchos, Anestis Hatzigeorgiadis and Konstantinos Boulas performed the operation. Basiliki Mauroeidi analyzed the imaging studies.
Contributor Information
Konstantinos Blouhos, Email: kostasblu@hotmail.com.
Konstantinos A. Boulas, Email: katerinantwna@hotmail.com.
Dimitrios G. Tselios, Email: dimtse@hotmail.com.
Stavroula P. Katsaouni, Email: skatsaouni1@yahoo.gr.
Basiliki Mauroeidi, Email: mavroeidi@gmail.com.
Anestis Hatzigeorgiadis, Email: ahatzigeorgiadis@gmail.com.
References
- 1.Tamm E.P., Balachandran A., Bhosale P.R., Katz M.H., Fleming J.B., Lee J.H. Imaging of pancreatic adenocarcinoma: update on staging/resectability. Radiologic Clinics of North America. 2012;50:407–428. doi: 10.1016/j.rcl.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Ishigami K., Yoshimitsu K., Irie H., Tajima T., Asayama Y., Nishie A. Diagnostic value of the delayed phase image for iso-attenuating pancreatic carcinomas in the pancreatic parenchymal phase on multidetector computed tomography. European Journal of Radiology. 2009;69:139–146. doi: 10.1016/j.ejrad.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 3.Goodman M., Willmann J.K., Jeffrey R.B. Incidentally discovered solid pancreatic masses: imaging and clinical observations. Abdominal Imaging. 2012;37:91–97. doi: 10.1007/s00261-011-9720-2. [DOI] [PubMed] [Google Scholar]
- 4.Yoon S.H., Lee J.M., Cho J.Y., Lee K.B., Kim J.E., Moon S.K. Small (≤20 mm) pancreatic adenocarcinomas: analysis of enhancement patterns and secondary signs with multiphasic multidetector CT. Radiology. 2011;259:442–452. doi: 10.1148/radiol.11101133. [DOI] [PubMed] [Google Scholar]
- 5.Kim J.H., Park S.H., Yu E.S., Kim M.H., Kim J., Byun J.H. Visually isoattenuating pancreatic adenocarcinoma at dynamic-enhanced CT: frequency, clinical and pathologic characteristics, and diagnosis at imaging examinations. Radiology. 2010;257:87–96. doi: 10.1148/radiol.10100015. [DOI] [PubMed] [Google Scholar]
- 6.Takeshita K., Kutomi K., Haruyama T., Watanabe A., Furui S., Fukushima J. Imaging of early pancreatic cancer on multidetector row helical computed tomography. British Journal of Radiology. 2010;83:823–830. doi: 10.1259/bjr/80905803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scaglione M., Pinto A., Romano S., Scialpi M., Volterrani L., Rotondo A. Using multidetector row computed tomography to diagnose and stage pancreatic carcinoma: the problems and the possibilities. Journal of the Pancreas. 2005;6:1–5. [PubMed] [Google Scholar]
- 8.Miura F., Takada T., Amano H., Yoshida M., Furui S., Takeshita K. Diagnosis of pancreatic cancer. HPB. 2006;8:337–342. doi: 10.1080/13651820500540949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wani S., Early D., Kunkel J., Leathersich A., Hovis C.E., Hollander T.G. Diagnostic yield of malignancy during EUS-guided FNA of solid lesions with and without a stylet: a prospective, single blind, randomized, controlled trial. Gastrointestinal Endoscopy. 2012;76:328–335. doi: 10.1016/j.gie.2012.03.1395. [DOI] [PubMed] [Google Scholar]
- 10.Thompson J.S., Murayama K.M., Edney J.A., Rikkers L.F. Pancreaticoduodenectomy for suspected but unproven malignancy. American Journal of Surgery. 1994;168:571–573. doi: 10.1016/s0002-9610(05)80124-2. [DOI] [PubMed] [Google Scholar]
- 11.Camp E.R., Vogel S.B. Blind Whipple resections for periampullary and pancreatic lesions. American Surgeon. 2004;70:6–10. [PubMed] [Google Scholar]
- 12.Garcea G., Metcalfe M.S., Berry D.P., Robertson G.S., Lloyd D.M., Dennison A.R. Is intraoperative confirmation of malignancy during pancreaticoduodenectomy mandatory? Journal of Gastrointestinal Surgery. 2012;16:370–375. doi: 10.1007/s11605-011-1728-y. [DOI] [PubMed] [Google Scholar]
- 13.Tummala P., Munigala S., Eloubeidi M.A., Agarwal B. Patients with obstructive jaundice and biliary stricture ± mass lesion on imaging: prevalence of malignancy and potential role of EUS-FNA. Journal of Clinical Gastroenterology. 2013:18. doi: 10.1097/MCG.0b013e3182745d9f. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]


