Abstract
Shoulder arthroplasty is considered the most effective surgical procedure for endstage shoulder pain from different causes including osteoarthritis, cuff-tear arthropathy, trauma, and tumors. Although uncommon and less frequent than knee or hip periprosthetic infection, periprosthetic shoulder infection represents a devastating complication and, despite treatment, is associated with unsatisfactory results. The most commonly identified microorganisms in periprosthetic shoulder infections are Staphylococcus aureus, coagulase-negative Staphylococci and Propionibacterium acnes. Diagnosis is not always easy and mainly derives from the integration of clinical symptoms, laboratory exams, radiological studies and microbiological swabs. Different options are available for treatment, including antibiotic therapy, lavage and debridement with retention of the prosthesis, one-stage reimplantation, two-stage reimplantation with antibiotic-impregnated cement spacer and resection arthroplasty. The aim of this review is to describe the current knowledge regarding risk factors, etiology, diagnosis and treatment of periprosthetic shoulder infection.
Keywords: Periprosthetic infections, shoulder arthroplasty, shoulder replacement.
INTRODUCTION
Shoulder arthroplasty is considered the most effective surgical procedure for endstage shoulder pain from different causes, including osteoarthritis, cuff-tear arthropathy, trauma, and tumors [1,2]. During the last decade the number of shoulder arthroplasty procedures more than doubled and it has been estimated that more than 46,000 shoulder replacements are performed annually in the United States [3]. In the majority of cases, this procedure provides significant pain relief and functional improvement with satisfactory longevity [4]. However, a number of potential complications may be associated with this surgery, the most frequent of which include implant loosening, shoulder instability, periprosthetic fracture, rotator cuff tear, nerve injury, deltoid dysfunction and infection [5].
PERIPROSTHETIC SHOULDER INFECTION
Although uncommon and less frequent than knee or hip periprosthetic infection [6], periprosthetic shoulder infection (PSI) remains a devastating complication and, despite treatment, is associated with unsatisfactory results [7]. The reported incidence of infection following primary anatomic total shoulder arthroplasty ranges between 0.4% to 2.9% but higher rates are estimated after revision surgery [8-10]. In contrast, the incidence of infection after primary reverse total shoulder arthroplasthy is around 5%, therefore considerably higher than after anatomic shoulder arthroplasty and comparable to knee or hip replacement [11]. The elevated infection rate of this kind of implant can be explained by the abundant dead space associated with the reverse configuration of the joint. In addition, in the absence of rotator cuff, the implant is not surrounded and protected by living tissue and it is therefore more susceptible to bacteria colonization [12].
RISK FACTORS
Patients with preexisting risk factors are more likely to acquire an infection after shoulder replacement [13,14]. Topolski et al. [15] showed in a study that 50% of the patients with a PSI had risk factors associated and Weber et al. [14] in a retrospective analysis found that all the patients in their study suffered from serious concomitant diseases. The majority of infections develop in presence of diabetes, systemic lupus erythematosus, rheumatoid arthritis, previous surgical procedures, and remote sources of infection. Other risk factors include chemotherapy, corticosteroid therapy and intrarticular steroid injections [5,16,17].
In addition, the risk of PSI increases if the surgical procedure is performed for fracture, cuff tear arthropathy, or osteonecrosis [2,7]. A previous study also reported that hematoma formation after should arthroplasty represented a risk factor for periprosthetic infection [10]. Finally, the axillary fossa includes hair follicles and sebaceous glands that facilitate the development of bacteria and thus the contamination of nearby surgical sites [18,19].
ETIOLOGY
The most commonly identified microorganisms in PSI are Staphylococcus aureus, coagulase-negative Staphylococcus and Propionibacterium acnes [2,10]. In particular, Propionibacterium acnes is an organism often found in PSI but hardly seen in periprosthetic hip or knee infections [20]. This organism, traditionally considered a contaminant more than a real pathogen, it is now recognized as a significant orthopaedic pathogen that has a peculiar propensity for the glenohumeral joint as it is the most common anaerobic germ isolated from normal skin in moist areas such as the axilla [13,18,21,22]. This slowly growing organism, typically responsible of late, chronic and relatively low-grade infections, can be difficult to isolate as it necessitates an extended culture time (8 days) to confirm a negative culture [21,23]. Polymicrobial infections are not rare and typically are caused by normal skin bacteria.
CLASSIFICATION
Classification of infection type after joint arthroplasty is important to choose the most suitable treatment. Some authors distinguish between infections caused by intraoperative wound contamination and haematogenous spread. Furthermore, there is a classification into superficial infections (restricted to the skin and subcutaneous tissue) and deep infections. Infections are divided into acute infection appearing 1–3 months after surgery, subacute infection appearing 4–12 months postoperatively, and late infection developing clinically later than 12 months postoperatively [8]. Fitzgerald et al. [24] classify infections into early infections, which appear in the postoperative stage, intermediate infections within 2–24 months after the operation, and late infections after more than 2 years. Acute infections in the postoperative stage are rare and mostly related to an infected wound haematoma or problems with the primary wound healing. These early infections can be treated most of the time with local wound care and i.v. antibiotics. Intermediate infections appear between the 2nd and 24th postoperative month. These infections usually require prosthesis removal. It is speculated that late postoperative infections after 2 or more years are frequently related to haematogenous spread of an infection to the implant. They often appear in patients with rheumatoid arthritis. If these infections are treated early and aggressively, there is a chance of healing without implant removal. If treatment is delayed, the process progresses and will require implant exchange. Other authors distinguish only between early and late infections. In particular, Harle distinguish between infections which appear up to the 6th postoperative week and infections which appear after more than 6 weeks. He argues that only a division into two infection types (early and late infections) is clinically relevant. He adds that the limit ought to be drawn soon (after 4–6 weeks) because only through early intervention in the first 6 weeks is there a real chance for salvaging the prosthesis [25]. The time interval from the diagnosis of infection to revision surgery and the therapy success rate correlate, so that early diagnosis and definitive treatment of the infection are important.
DIAGNOSIS
An early detection and a successful treatment can help to stop the development of longterm infection and significant impairment of bone and soft tissue [26]. On the contrary, a delay in diagnosing a PSI can result in long-term pain, prosthetic instability and sometimes sepsis. Diagnosis derives from the integration of clinical symptoms, laboratory exams, radiological studies and microbiological swabs. However, diagnosing a PSI is not always easy as symptoms may be minimal. Moreover, the inactive disposition of common shoulder pathogens can hinder the diagnosis of shoulder pathogens like Propionibacterium acnes. An infected shoulder prosthesis is less disabling than an infected hip or knee prosthesis, because scapulothoracic movement is preserved and the arm needs to bear no weight.
Clinical symptoms.
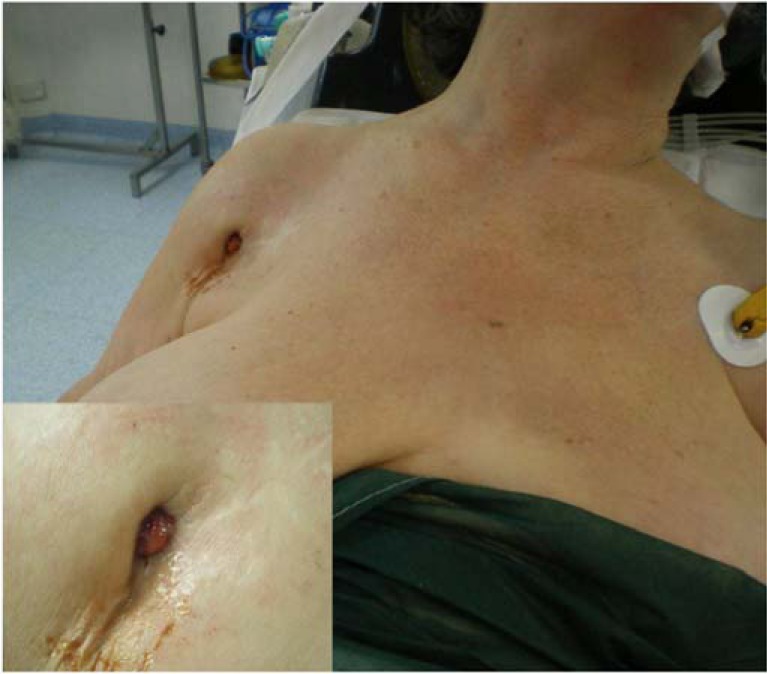
The clinical presentation of PSI is generally nonspecific and the most frequent symptom to be presented is that of pain, whereas other clinical signs of infection may be lacking. The existence of a sinus tract that connects with the prosthesis is definitive proof of PSI (Fig. 1). Another local sign that may be found is erythema, while systemic symptoms (fever, chills) are less common [19]. The Infectious Diseases Society of America recommend considering a periprosthetic infection in presence of “an acute onset of a painful prosthesis, or any chronic painful prosthesis at any time after prosthesis implantation, especially when there are no pain-free periods, in the first few years following implantation or if there is a history of prior wound healing problems” [27]. Treatment using nonsteroidal anti-inflammatory medications and oral antibiotics can impede a correct diagnosis [19].
Fig. (1).
A draining sinus tract in the medial aspect of the deltopectoral surgical incision.
Laboratory tests.
Laboratory tests, including analyisis of the C-reactive protein (CRP) level, erythrocyte sedimentation rate (ESR), and white bloodcell count (WBC) are significant signs of infection [16,17]. The WBC count in a deep infection is seldom abnormal, while the ESR and CRP level are frequently elevated [7,19,28]. Nevertheless, the ESR and CRP level are aspecific markers of inflammation and could be unremarkable in cases of Propionibacterium acnes infection [21]. Moreover, both markers are normally higher following an uncomplicated surgical operation. The ESR will fall gradually, while the CRP level will diminish faster, typically returning to normal levels inside two weeks after a routine operation. Topolski et al. [15] described the value of pre-operative studies and intra-operative histology in patients who underwent revision shoulder replacement and had positive intraoperative cultures. In a series of 75 shoulders without obvious signs of infection undergoing revision, the pre-operative WBC count was normal in 67 of 72, with normal polymorphonuclear cell distributions and a normal ESR in most patients. Among the 16 patients who had a CRP measured, it was normal in 12. The intra-operative histology showed no signs of acute inflammation in 67 of 73 patients. The authors concluded that there were no reliable intra- or pre-operative studies to forecast who was likely to have a positive intra-operative culture.
Radiological findings.
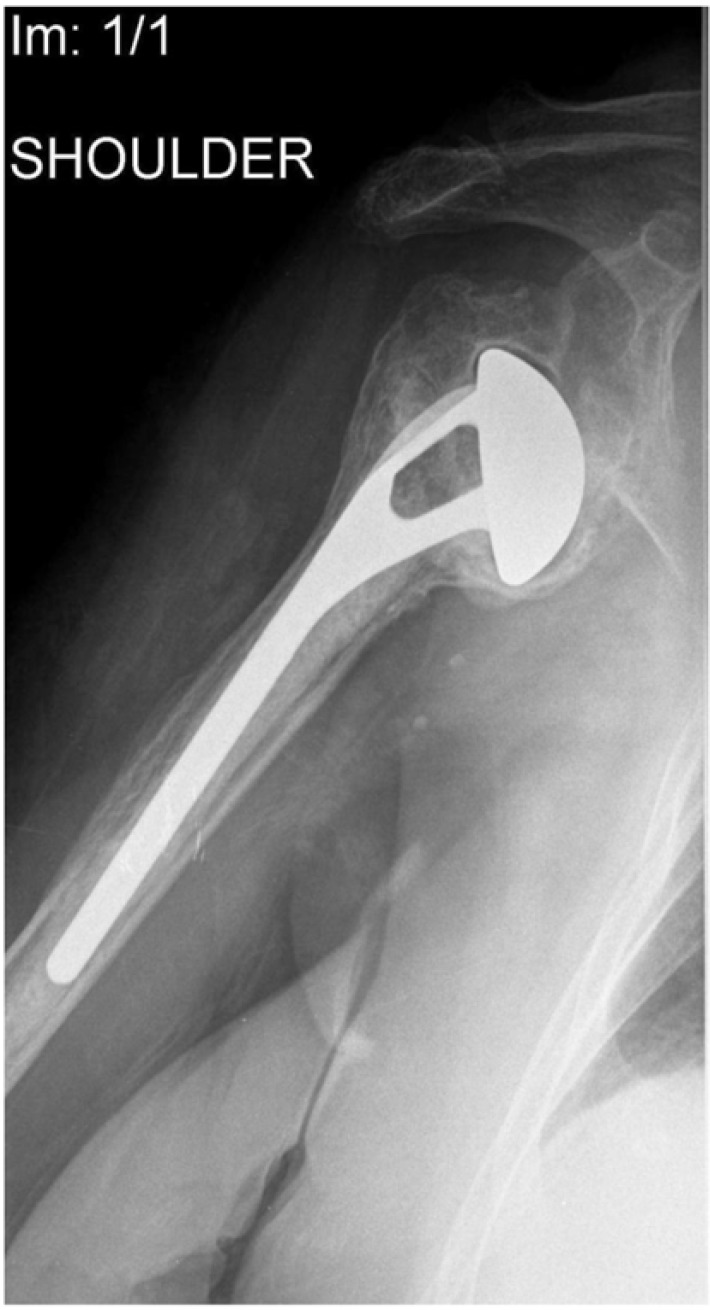
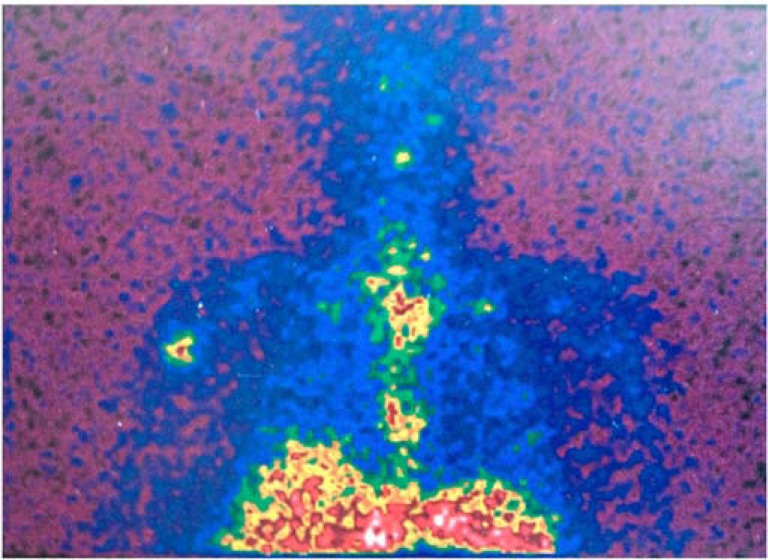
With early infection, radiographic findings are typically normal. In contrast, radiographs may provide important clues in subacute and chronic infections. Lucent lines, which develop within a few months, strongly signal an infection (Fig. 2). Other subtle radiological findings include the presence of medial calcar erosion and resorption of the tuberosities. Ultrasonography may be useful identifying localized fluid accumulation as some PSI could present with fluid collections which can be traced to remote locations from the glenohumeral joint [29]. Technetium Tc-99 bone scans and indium In-111–labeled white blood cell scintigraphy should be taken into account when the diagnosis of PSI continues to be in doubt. (Fig. 3) [7].
Fig. (2).
Radiograph of a shoulder 1 year after hemiarthroplasty. Note a lucent periprosthetic line at the bone-prosthesis interface with signs of loosening.
Fig. (3).
Labeled leukocyte scintigraphy revealing an increased uptake localized in the proximal periprosthetic zone of the humerus.
Microbiological and histological studies.
All patients with a suspected PSI should have a diagnostic arthrocentesis carried out except where the disgnosis is clinically obvious and surgery is planned. It is recommended that a synovial fluid analysis contains a total cell count and differential leukoyte count, along with culture for aerobic and anerobic organisms. A white blood cell count of >50,000 cells/mm3 with more than 75% polymorphonuclear cells or even direct germ visualization are suggestive of infection. Withholding antibiotic treatment for a minimum of two weeks before the arthrocentesis enhances the chances of retrieving an organism. Intraoperative histopathological examination of periprosthetic tissue samples is a well proven diagnostic test provided that a skilled pathologist specialized in the evaluation of periposthetic tissue is to hand. It should be carried out during the surgery, if the presence of infection is in question, based on the clinical judgement of the surgeon and the results will determine the choice of treatment. A minimum of four periprosthetic intraoperative tissue samples of the explanted prosthesis should be sent for aerobic and anaerobic culture during the surgery in order to increase the possibility of getting a microbiologic diagnosis: two specimens from the joint capsule, one from the prosthesis-bone interface and another from the medulary canal. More than five polymorphonuclear leukoytes per high-powered field signify infection when they are found in a minimum of two tissue specimens.. Withholding antibiotic treatment for a minimum of 2 weeks before collecting intraoperative culture enhances the chances of retrieving an organism. For the same reason, perioperative antibiotics should be withheld until tissue samples are obtained [27]. Implant sonication is a recent technique that involves the removal of the prosthesis and putting it into a solid container which is then vortexed and sonicated. The technique can be used to identify germs in the biofilm clinging to the prosthesis. Implant sonication has proven to be more sensitive than intraoperative tissue specimens for identification of bacteria, especially Propionibacterium acnes: although the test has yet to obtain extensive acceptance [30].
TREATMENT
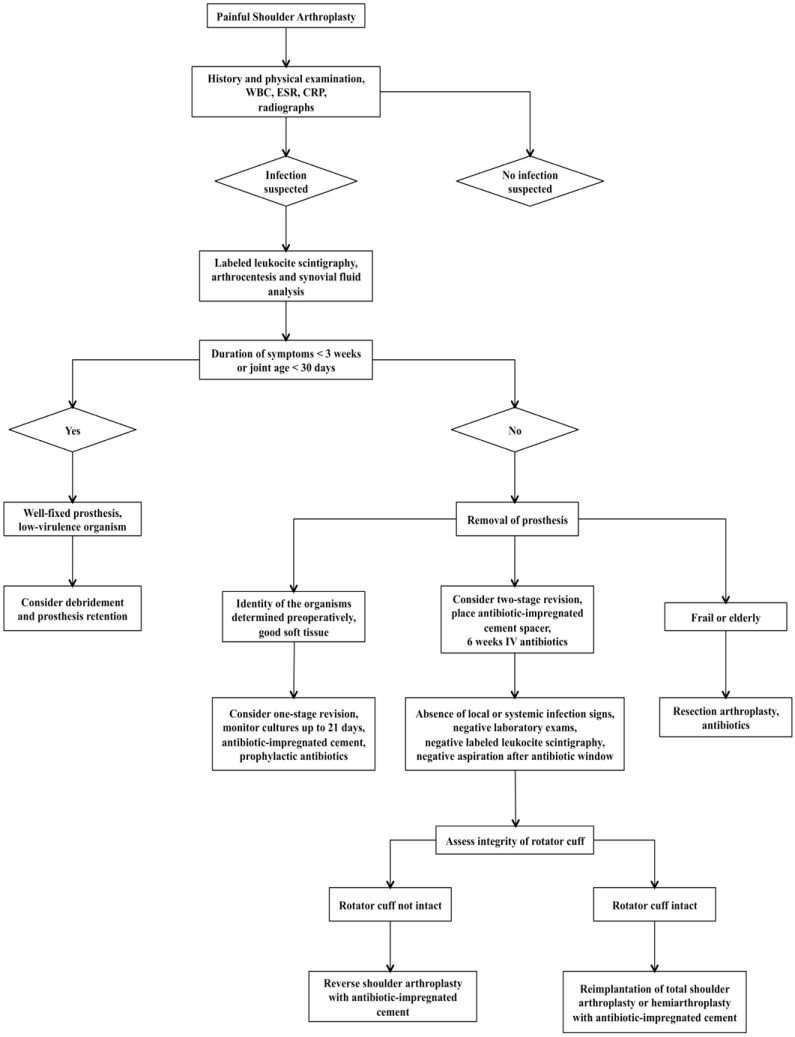
The treatment strategies for a PSI are similar to those used for other periprosthetic joint infections and include antibiotic therapy, debridement with retention of the prosthesis, direct prosthesis exchange (one-stage exchange), staged prosthesis exchange with temporary placement of an antibiotic-impregnated spacer (two-stage exchange) and resection arthroplasty. In each case, the choice of treatment is dependent on the virulence of the pathogens, sensitivity to antibiotics, stability of the implant, and the time interval between primary implantation and clinical manifestation of the infection. The treatment algorithms for PSI (Fig. 4) at the present time reflect the standard protocols for periprosthetic hip and knee infections.
Fig. (4).
The treatment algorithms for PSI currently mirror those of protocols established for infections associated with hip and knee arthroplasties.
Antibiotic Therapy.
Antibiotic therapy was demonstrated to be ineffective in the management of PSI, confirming the results of antibiotic suppression for periprosthetic knee infections. Failure rates of 60% to 75% have been reported with this treatment modality [7,31]. Antibiotic treatment alone should only be contemplated for seriously ill patients or those who refuse to have surgery. [19].
Debridement with prosthesis retention.
Retention of a shoulder prosthesis can be feasible when an infection is identified acutely (after less than 30 days from implantation or after less than three weeks from the start of infectious symptoms), the implant is stable, the isolated germ is of low virulence, and complete debridement has been achieved [19]. In selected cases, debridement may also be performed arthroscopically [32]. However, Sperling et al. [13] reported a 50% failure rate with this treatment and Romanò et al. [20] in a multicentric study reported less satisfactory results with debridement in terms of infection eradication rate compared to the other treatments. Coste et al. [7] have carried out a retrospective review of 49 cases of PSI and concluded that antibiotics or debridement alone were not effective. They suggested to treat acute PSI with aggressive debridement and exchange of the implant associated with an appropriate intravenous antibiotic therapy.
One-stage exchange.
Formed mainly from the experiences gained in hip and knee surgery, one-stage prosthesis exchange is not as popular as two-stage prosthesis exchange. Sperling et al. [13] reported failure in one of two shoulders treated for subacute PSI with direct prosthesis exchange. On the contrary, Coste et al. [7] detected improvement in Constant scores and no recurrence of infection, in 3 patients treated with one-stage prosthesis exchange. Ince et al. [33] also reported no recurrence of infection in 16 patients managed with one-stage revision using an antibiotic-impregnated cement. Despite the fact that two-stage exchange is generally regarded as the gold standard, Cuff et al. [34] in a retrospective analysis of 22 cases reported that, as regards outcome and the eradication of infection, a single-stage revision was statistically no different from a two-stage procedure. The one-stage reimplantation guarantees better functional outcome but carries a higher risk of infection recurrence. Other benefits of one-stage exchange over a two-stage exchange involve lower cost and the requirement for only one procedure. Nontheless, failure to eradicate the infection during the one-stage reimplantation, will require additional procedures. More investigation is needed to establish guidelines and identify the appropriate conditions for using a one-stage exchange [19].
Two-stage exchange.
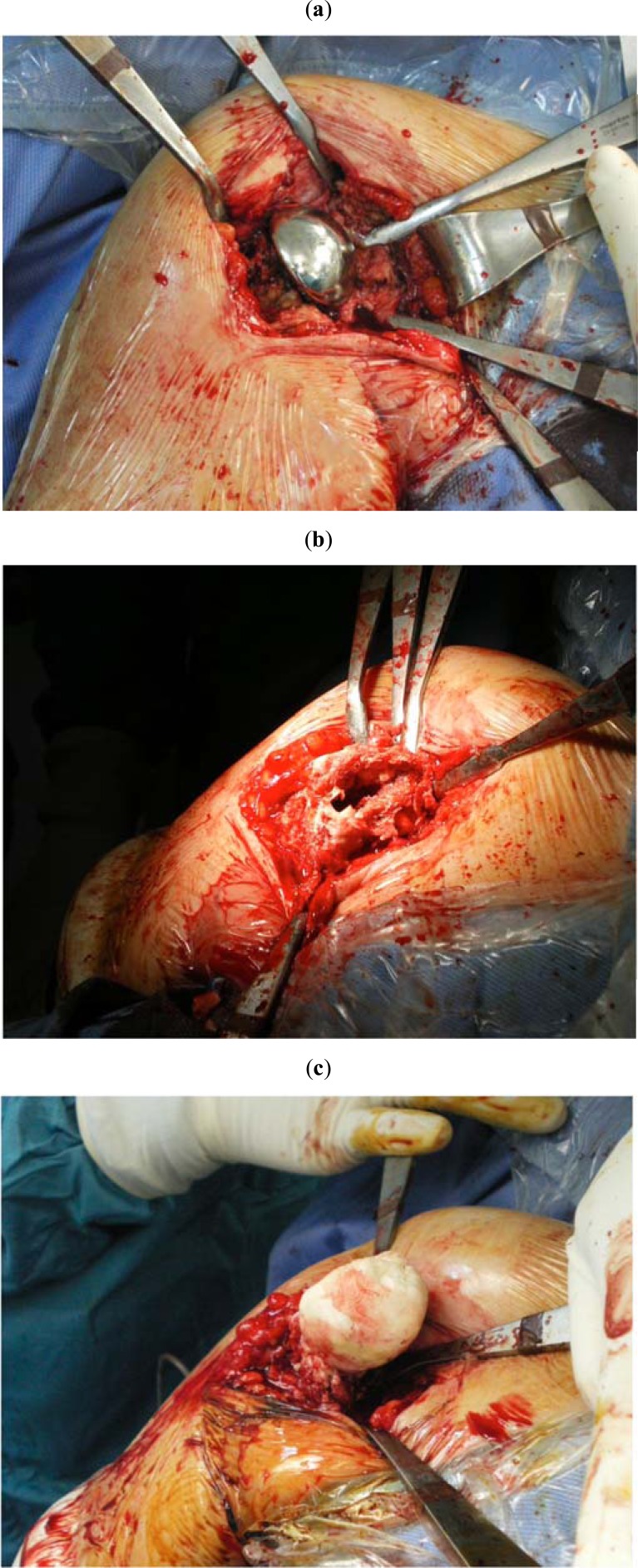
Two-stage exchange involves removal of the infected implant, extensive debridement of periprosthetic tissues, placement of a temporary antibiotic spacer (Fig. 5a-c) and antibiotic treatment prior to reimplantation of new prosthetic components. Different authors have suggested to discontinue antibiotics and monitorize the level of CRP and ESR prior to going ahead with revision surgery [19]. One-stage and two-stage reimplantation show high rates of infection control, although the most reproducible results have been gotten from two-stage revision surgery [8,26]. Moreover, the two-stage reimplantation appears to be able to compromise the best between dependable eradication of the infection and functionality after surgery. Sperling et al. [13] examined 32 PSI cases: group I (21 cases) underwent resection arthroplasty; group II (6 cases), underwent debridement and retention of the implant; group III (2 cases) was treated with one-stage exchange; and group IV (3 cases), with two-stage exchange. Reinfection occurred in 6 patients treated with resection arthroplasty, in 3 patients with the retained implant, in 1 patient treated with one-stage reimplantation, and in none of the patients treated with two-stage reimplantation. At the time of final follow-up, the authors concluded that two-stage exchange gives the best results in terms of eradication of infection, pain relief, and shoulder function, particularly if compared to resection arthroplasty. Sabesan et al. [26] also reported a low recurrence rate after a two-stage reimplantation procedure associated with a substantial improvement in function and pain. Strickland et al. [35] describes less successful outcomes with two-stage exchange. In their group of 19 patients, the eradication of infection was obtained in only 63% of cases (12), and in 13 cases the outcomes were unsatisfactory. Antibiotic-impregnated cement spacers have proven to be of use in staged reimplantation [36,37]. The spacer maintains soft-tissue tension, reduces pain, enhances the patients functional status and enables the patient to do physical therapy prior to reimplantation of a prosthesis. The spacer permits local antibiotic administration and induces the development of a pseudocapsule that can be mobilized with the cuff during ensuing surgeries. In a recent study, 9 of 11 patients who had a spacer implanted for sepsis or PSI were satisfied and put off the remotion of the spacer. Also other authors support prolonged or even permanent retention of the spacer as a possible substitute to reimplantation in complicated patients who are at increased surgical risk. Finally, a viable option for patients with cuff-tear arthropathy and a working deltoid, is the reverse shoulder arthroplasty. Reverse shoulder arthroplasty could permit improved function in patients who have undergone debridement of periprosthetic tissues. In the case of infection, the quality of debridement is deemed a crucial factor for the eradication of infection. An aggressive debridement of suspicious soft and bone tissue can be carried out with less apprehension for decreased functionality when a reverse shoulder replacement is going to be implanted [26,34].
Fig. (5).
Intraoperative images of an infected shoulder arthroplasty (a) before the removal of the prosthesis, (b) after performing irrigation and debridement and (c) after the implantation of an antibiotic-impregnated cement spacer.
Resection arthroplasty.
One-stage and two-stage reimplantation does give the greatest chance of restoring function, but in some cases this may not be possible. In the elderly, debilitated patients, with high-virulence germs, significant loss of soft tissue, or poor health, a reimplantation may be unwise. In these conditions, resection arthroplasty represents an acceptable solution. It offers the opportunity to obtain the eradication of infection and good pain relief with just one surgical procedure with the only downside being limited function [38]. Many studies have indicated that resection arthroplasty provides excellent eradication rates, although, often at the price of significant functional deficits [14]. In particular it is the loss of internal and external rotation that hinder the patients during activities of daily living. [36,39]. It is therefore generally accepted to be a treatment for older, low demand patients. Codd et al. [40] compared the treatment results with infected shoulder prostheses after resection arthroplasty and prosthesis reimplantation. Pain reduction was achieved with approximately the same efficacy with both joint resection and prosthesis revision. The patients who had resection arthroplasty, however, had significantly reduced shoulder motion and difficulties with every-day activities. Rispoli et al. [41] reported substantial functional impairments in spite of good pain relief in 18 patients treated with resection arthroplasty for PSI or failed shoulder replacement.
CONCLUSIONS
PSI is a rare but catastrophic complication that continues to be both a diagnostic and treatment challenge. Patients who develop a PSI will have worse outcomes compared to patients who go through an uncomplicated procedure even if appropriately treated. Preventive measures are then of primary importance and include adequate surgical site preparation with an effective antiseptic, prophylactic antibiotic intraoperative administration, preoperative blood glucose control for patients with diabetes, minimize the use of immunosuppressive medication whenever is possible and short operating times.
ACKNOWLEDGEMENTS
The authors wish to thank Mrs Sharon Dehmel for reviewing the English.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Chillemi C, Franceschini V. Shoulder osteoarthritis. Arthritis [serial on the internet] Available from http://www.hindawi.com/journals/arth/2013/370231/ 2013. Jan 10, [DOI] [PMC free article] [PubMed]
- 2.Singh JA, Sperling JW, Schleck C, Harmsen W, Cofield RH. Periprosthetic infections after shoulder hemiarthroplasty. J Shoulder Elbow Surg. 2012;21(10):1304–9. doi: 10.1016/j.jse.2011.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249–54. doi: 10.2106/JBJS.J.01994. [DOI] [PubMed] [Google Scholar]
- 4.Izquierdo R, Voloshin I, Edwards S, et al. Treatment of glenohumeral osteoarthritis. J Am Acad Orthop Surg. 2010;18(6):375–82. doi: 10.5435/00124635-201006000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Bohsali KI, Wirth MA, Rockwood CA., Jr Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279–92. doi: 10.2106/JBJS.F.00125. [DOI] [PubMed] [Google Scholar]
- 6.Fehringer EV, Mikuls TR, Michaud KD, Henderson WG, O'Dell JR. Shoulder arthroplasties have fewer complications than hip or knee arthroplasties in US veterans. Clin Orthop Relat Res. 2010;468(3):717–22. doi: 10.1007/s11999-009-0996-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coste JS, Reig S, Trojani C, Berg M, Walch G, Boileau P. The management of infection in arthroplasty of the shoulder. J Bone Joint Surg Br. 2004;86(1):65–69. [PubMed] [Google Scholar]
- 8.Jerosch J, Schneppenheim M. Management of infected shoulder replacement. Arch Orthop Trauma Surg. 2003;123(5):209–14. doi: 10.1007/s00402-003-0497-9. [DOI] [PubMed] [Google Scholar]
- 9.Boileau P, Sinnerton RJ, Chuinard C, Walch G. Arthroplasty of the shoulder. J Bone Joint Surg Br. 2006;88(5):562–75. doi: 10.1302/0301-620X.88B5.16466. [DOI] [PubMed] [Google Scholar]
- 10.Cheung EV, Sperling JW, Cofield RH. Infection associated with hematoma formation after shoulder arthroplasty. Clin Orthop Relat Res. 2008;466:1363–7. doi: 10.1007/s11999-008-0226-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farshad M, Gerber C. Reverse total shoulder arthroplasty-from the most to the least common complication. Int Orthop. 2010;34(8):1075–82. doi: 10.1007/s00264-010-1125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Cupis V, Chillemi C, Marinelli M. Grammont inverted prosthesis for the treatment of cuff tear arthropathy: a 6-year follow-up study. Orthopedics. 2008;31(5):447. doi: 10.3928/01477447-20080501-06. [DOI] [PubMed] [Google Scholar]
- 13.Sperling JW, Kozak TK, Hanssen AD, Cofield RH. Infection after shoulder arthroplasty. Clin Orthop Relat Res. 2001;382:206–16. doi: 10.1097/00003086-200101000-00028. [DOI] [PubMed] [Google Scholar]
- 14.Weber P, Utzschneider S, Sadoghi P, Andress HJ, Jansson V, Müller PE. Management of the infected shoulder prosthesis: a retrospective analysis and review of the literature. Int Orthop. 2011;35(3):365–73. doi: 10.1007/s00264-010-1019-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Topolski MS, Chin PY, Sperling JW, Cofield RH. Revision shoulder arthroplasty with positive intraoperative cultures: the value of preoperative studies and intraoperative histology. J Shoulder Elbow Surg. 2006;15(4):402–6. doi: 10.1016/j.jse.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Matsen FA, 3rd, Rockwood CA, Jr, Wirth MA, Lippitt SB, Parsons M. Glenohumeral arthritis and its management. In: Rockwood CA Jr, Matsen FA 3rd, Wirth MA, Lippitt SB, editors. The Shoulder. 3rd ed. Philadelphia: WB Saunders; 2004. [Google Scholar]
- 17.Wirth MA, Rockwood CA., Jr Complications of total shoulder-replacement arthroplasty. J Bone Joint Surg Am. 1996;78(4):603–16. doi: 10.2106/00004623-199604000-00018. [DOI] [PubMed] [Google Scholar]
- 18.Patel A, Calfee RP, Plante M, Fischer SA, Green A. Propionibacterium acnes colonization of the human shoulder. J Shoulder Elbow Surg. 2009;18(6):897–902. doi: 10.1016/j.jse.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 19.Saltzman MD, Marecek GS, Edwards SL, Kalainov DM. Infection after shoulder surgery. J Am Acad Orthop Surg. 2011;19(4):208–18. doi: 10.5435/00124635-201104000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Romanò CL, Borens O, Monti L, Meani E, Stuyck J. What treatment for periprosthetic shoulderinfection? Results from a multicentre retrospective series. Int Orthop. 2012;36(5):1011–7. doi: 10.1007/s00264-011-1467-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dodson CC, Craig EV, Cordasco FA, et al. Propionibacterium acnes infection after shoulder arthroplasty: a diagnostic challenge. J Shoulder Elbow Surg. 2010;19(2):303–7. doi: 10.1016/j.jse.2009.07.065. [DOI] [PubMed] [Google Scholar]
- 22.Singh JA, Sperling JW, Schleck C, Harmsen WS, Cofield RH. Periprosthetic infections after total shoulder arthroplasty: a 33-year perspective. J Shoulder Elbow Surg. 2012;21(11):1534–41. doi: 10.1016/j.jse.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeller V, Ghorbani A, Strady C, Leonard P, Mamoudy P, Desplaces N. Propionibacterium acnes: an agent of prosthetic joint infection and colonization. J Infect. 2007;55(2):119–24. doi: 10.1016/j.jinf.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Fitzgerald RH, Jr, Bechtol CO, Eftekhar N, Nelson JP. Reduction of deep sepsis after total hip arthroplasty. Arch Surg. 1979;114(7):803–4. doi: 10.1001/archsurg.1979.01370310045009. [DOI] [PubMed] [Google Scholar]
- 25.Härle A. Infection management in total hip replacement. Arch Orthop Trauma Surg. 1989;108(2):63–71. doi: 10.1007/BF00932159. [DOI] [PubMed] [Google Scholar]
- 26.Sabesan VJ, Ho JC, Kovacevic D, Iannotti JP. Two-stage reimplantation for treating prosthetic shoulder infections. Clin Orthop Relat Res. 2011;469(9):2538–43. doi: 10.1007/s11999-011-1774-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the infectious diseases society of america. Clin Infect Dis. 2013;56(1):e1–e25. doi: 10.1093/cid/cis803. [DOI] [PubMed] [Google Scholar]
- 28.Themistocleous G, Zalavras C, Stine I, Zachos V, Itamura J. Prolonged implantation of an antibiotic cement spacer for management of shoulder sepsis in compromised patients. J Shoulder Elbow Surg. 2007;16(6):701–5. doi: 10.1016/j.jse.2007.02.118. [DOI] [PubMed] [Google Scholar]
- 29.Potter HG, Schweitzer ME, Altchek DW. Advanced imaging in orthopaedics: current pitfalls and new applications. Instr Course Lect. 1997;46:521–9. [PubMed] [Google Scholar]
- 30.Portillo ME, Salvadó M, Sorli L, et al. Multiplex PCR of sonication fluid accurately differentiates between prosthetic joint infection and aseptic failure. J Infect. 2012;65(6):541–8. doi: 10.1016/j.jinf.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 31.Sperling JW, Galatz LM, Higgins LD, Levine WN, Ramsey ML, Dunn J. Avoidance and treatment of complications in shoulder arthroplasty. Instr Course Lect. 2009;58:459–72. [PubMed] [Google Scholar]
- 32.Jeon IH, Choi CH, Seo JS, Seo KJ, Ko SH, Park JY. Arthroscopic management of septic arthritis of the shoulder joint. J Bone Joint Surg Am. 2006;88(8):1802–6. doi: 10.2106/JBJS.E.00917. [DOI] [PubMed] [Google Scholar]
- 33.Ince A, Seemann K, Frommelt L, Katzer A, Loehr JF. One stage exchange shoulder arthroplasty for peri-prosthetic infection. J Bone Joint Surg Br. 2005;87(6):814–8. doi: 10.1302/0301-620X.87B6.15920. [DOI] [PubMed] [Google Scholar]
- 34.Cuff DJ, Virani NA, Levy J, et al. The treatment of deep shoulder infection and glenohumeral instability with debridement, reverse shoulder arthroplasty and postoperative antibiotics. J Bone Joint Surg Br. 2008;90(3):336–42. doi: 10.1302/0301-620X.90B3.19408. [DOI] [PubMed] [Google Scholar]
- 35.Strickland JP, Sperling JW, Cofield RH. The results of two-stage re-implantation for infected shoulder replacement. J Bone Joint Surg Br. 2008;90(4):460–5. doi: 10.1302/0301-620X.90B4.20002. [DOI] [PubMed] [Google Scholar]
- 36.Verhelst L, Stuyck J, Bellemans J, Debeer P. Resection arthroplasty of the shoulder as a salvage procedure for deep shoulder infection: does the use of a cement spacer improve outcome? J Shoulder Elbow Surg. 2011;20(8):1224–33. doi: 10.1016/j.jse.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 37.Proubasta IR, Itarte JP, Lamas CG, Escribá IU. Permanent articulated antibiotic-impregnated cement spacer in septic shoulder arthroplasty: a case report. J Orthop Trauma. 2005;19(9):666–8. doi: 10.1097/01.bot.0000153448.80988.dc. [DOI] [PubMed] [Google Scholar]
- 38.Braman JP, Sprague M, Bishop J, Lo IK, Lee EW, Flatow EL. The outcome of resection shoulder arthroplasty for recalcitrant shoulder infections. J Shoulder Elbow Surg. 2006;15(5):549–53. doi: 10.1016/j.jse.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 39.Debeer P, Plasschaert H, Stuyck J. Resection arthroplasty of the infected shoulder: a salvage procedure for the elderly patient. Acta Orthop Belg. 2006;72(2):126–30. [PubMed] [Google Scholar]
- 40.Codd TP, Yamaguchi K, Pollock RG, Flatow EL, Bigliani LU. Infected shoulder arthroplasties: treatment with staged reimplantation vs resection arthroplasty. Orthop Trans. 1996;20:59. [Google Scholar]
- 41.Rispoli DM, Sperling JW, Athwal GS, Schleck CD, Cofield RH. Pain relief and functional results after resection arthroplasty of the shoulder. J Bone Joint Surg Br. 2007;89(9):1184–7. doi: 10.1302/0301-620X.89B9.19464. [DOI] [PubMed] [Google Scholar]