Abstract
BACKGROUND
Moderate (approximately 2-fold) increases in plasma unconjugated bilirubin levels are able to attenuate the development of angiotensin II (Ang II)–dependent hypertension. To determine the specific role of decreases in superoxide production to the blood pressure–lowering effects of moderate hyperbilirubinemia (MHyB), we performed this study, in which the Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase inhibitor apocynin was given to Ang II–infused mice in the presence and absence of moderate hyperbilirubinemia.
METHODS
Apocynin (14mM) was administered in the drinking water prior to treatment with UDP-glucuronosyltransferase 1A1 antisense morpholino (16 μg/kg), which was administered by intravenous injection every third day. Treatments were started before the implantation of Ang II–containing minipumps (1μg/kg/min) and continued throughout the protocol.
RESULTS
Ang II infusion increased blood pressure to 145±2mm Hg. Apocynin treatment alone reduced blood pressure to 135±5mm Hg, whereas MHyB alone decreased blood pressure to 118±5mm Hg in Ang II–infused mice. Prior inhibition of NADPH oxidase with apocynin did not result in a further decrease in blood pressure in MHyB mice, which averaged 117±3mm Hg (n = 6 mice per group). In aortic preparations, apocynin treatment decreased Ang II–mediated superoxide production from 2433±120 relative light units (RLU)/min/mg to 1851±126 RLU/min/mg (n = 4 mice per group), which was similar to levels observed in MHyB mice alone (1473±132 RLU/min/mg) or in combination with apocynin (1503±115 RLU/min/mg).
CONCLUSIONS
Our results indicate that MHyB lowers blood pressure by a mechanism that is partially dependent on the inhibition of superoxide production.
Key Words: angiotensin II, bilirubin, blood pressure, heme oxygenase, hypertension, NADPH oxidase
Bilirubin in the serum is derived from the breakdown of red blood cells in the spleen. Heme, which is a breakdown product of red blood cells, is metabolized to iron, carbon monoxide, and biliverdin by heme oxygenase enzymes in the spleen. Biliverdin is then converted to bilirubin by the enzyme biliverdin reductase. Bilirubin is then bound to albumin and transported in the blood to hepatocytes of the liver to be conjugated for elimination in the bile. In the liver, UDP-glucuronosyltransferase 1A1 (UGT1A1) is the enzyme responsible for the conjugation of bilirubin, which is transported in the bile for elimination. Alterations in UGT1A1 that decrease the conjugation result in increased levels of unconjugated bilirubin in the serum and are responsible for Gilbert’s syndrome. Several large population studies have established that moderate increases in serum bilirubin levels (approximately 2-fold) are negatively associated with the development of cardiovascular disease.1,2 The same relationship was also found in patients with Gilbert’s syndrome.3,4 We have previously reported that similar moderate increases in plasma bilirubin achieved by antagonism of UGT1A1 with indinavir or knockdown of UGT1A1 protein with antisense (AS) morpholinos can attenuate the increase in blood pressure and decrease in renal blood flow and glomerular filtration rate in angiotensin II (Ang II)–dependent hypertension in mice.5,6
Bilirubin is one of the most potent endogenous antioxidants in the body.7,8 It is hypothesized that the antioxidant actions of bilirubin may be responsible for the protective actions against cardiovascular disease in patients with Gilbert’s syndrome.9 Induction of moderate hyperbilirubinemia in our mouse model was associated with a decrease in Ang II–dependent superoxide production in the aorta.5 Bilirubin can not only act as a scavenger but can also inhibit NADPH oxidase enzymes to directly decrease the formation of superoxide anion.10 Because NADPH-mediated superoxide production has been previously demonstrated to play a critical role in the development and maintenance of Ang II–dependent hypertension,11,12 we wanted to test the hypothesis that attenuation of superoxide production mediates the antihypertensive actions of moderate hyperbilirubinemia in Ang II–dependent hypertension. In order to accomplish this, we determined the effect of moderate hyperbilirubinemia on Ang II–dependent hypertension in mice in which NADPH oxidase activity was decreased using the NADPH oxidase inhibitor apocynin.
METHODS
Animals
Experiments were performed on 12- to 16-week-old male C57BL/6J mice obtained from Jackson Labs (Bar Harbor, ME). The mice were fed a standard diet containing 0.29% sodium chloride and were provided water ad libitum. All animal protocols were approved by the Institutional Animal Care and Use Committee at the University of Mississippi Medical Center and performed in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Mice were randomly assigned to 1 of 4 experimental groups: (ii) Ang II treatment, (ii) Ang II + apocynin treatment, (iii) Ang II + UGT1A1 AS treatment, (iv) Ang II + apocynin + UGT1A1 AS treatment. All mice underwent surgery for implantation of jugular vein catheters. Mice that received apocynin (14mM in water supplemented with 5% sucrose) were started on the drug 2 days before venous catheter surgery. Apocynin dosing was based on previously published studies in mice.13,14 Mice were then treated with intravenous infusion of saline or UGT1A1 AS morpholino oligonucleotide (16 μg/kg, Vivo morpholinos, AGCTCCAGCACACCACAGTCATGGT; Gene Tools, Philomath, OR) every third day throughout the entire experimental protocol. Mice received 1 AS treatment before implantation of Ang II–containing (1 μg/kg/min) osmotic minipumps implanted subcutaneously in mice under light isoflurane anesthesia, as previously reported.15 Five days after implantation of the osmotic minipumps, carotid artery catheters were implanted, as previously reported.15 After a 48-hour recovery period, blood pressure was measured in conscious, freely moving mice in their home cage for 3 hours each morning over the next 3 days. Mice were killed at the end of the experimental protocol, at which time body weight and heart weight were recorded.
Measurement of plasma bilirubin
Plasma samples were collected from mice of each experimental group at the end of the experimental protocol. Mice were killed by carbon dioxide asphyxiation, and the heart was immediately removed. Pooled whole blood was then collected from the chest cavity and placed in tubes containing 5 μl of an Ethylenediaminetetraacetic acid (EDTA) solution (0.5M). The blood was then centrifuged at 3,000 g for 5 minutes, and plasma was collected and stored at −20 °C. Total bilirubin and conjugated bilirubin concentrations were measured from 150 μl using the QuantiChrom Bilirubin Assay Kit (BioAssay Systems, Hayward, CA) according to the manufacturer instructions. The bilirubin assay was calibrated with a solution equivalent to 5mg/dl and provided by the manufacturer. Unconjugated bilirubin was calculated as the difference between total bilirubin and conjugated bilirubin. The concentrations are expressed as milligrams per deciliter.
Glomerular filtration rate (GFR)
The GFR was measured by continuous infusion of fluorescein isothiocyanate (FITC)–labeled inulin on days 5 and 6 after implantation of Ang II osmotic minipump, as previously described. FITC-labeled inulin was infused intravenously at a rate of 10.5 μg/min for 24 hours to reach steady state. Once steady state is achieved, the infusion rate of FITC-labeled inulin is equal to the urinary excretion rate. An arterial plasma sample (25 μl) was collected by retro-orbital bleed in isoflurane-anesthetized mice, and 5 μl was measured with a microplate fluorometer (Bio Tek Instruments, Winooski, VT). Two consecutive GFR measurements were averaged for each individual mouse and expressed as milliliters per minute per gram kidney weight (KW).
Measurement of vascular superoxide
Superoxide production in the aorta was measured using the lucigenin technique, as previously described.16,17 Briefly, aortas were removed and separated from perivascular adipose tissue and snap frozen in liquid nitrogen and stored at −80 °C. The aortas were then homogenized (1:8 wt/vol) in Radio-Immunoprecipitation Assay (RIPA) buffer (phosphate-buffered saline, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, and a protease inhibitor cocktail; Sigma Chemical, St. Louis, MO). The samples were centrifuged at 12,000 g for 20 minutes at 4 °C. The supernatant was incubated with lucigenin at a final concentration of 5 µM and NADPH at a final concentration of 100 μM. The samples were allowed to equilibrate for 3 minutes in the dark, and luminescence was measured every second for 5–15 minutes with a luminometer (Berthold, Oak Ridge, TN). Luminescence was recorded as relative light units (RLU) per minute. An assay blank with no homogenate but containing lucigenin was subtracted from the reading before transformation of the data. The protein concentration was measured using a Bio-Rad protein assay (BioRad, Hercules, CA) with bovine serum albumin (BSA) standards. The data are expressed as relative light units per minute per milligram protein.
Statistics
Mean values ± SEMs are presented. Significant differences between mean values were determined by analysis of variance followed by a post hoc test (Dunnett’s). P < 0.05 was considered to be significant.
RESULTS
Treatment with UTG1A1 AS morpholino increased levels of unconjugated bililrubin in Ang II– and Ang II + apocynin–treated mice
The levels of total bilirubin, direct bilirubin, and unconjugated bilirubin were identical in Ang II– and Ang II + Apocynin–treated mice (Table 1). Treatment with UGT1A1 AS resulted in increases in total bilirubin and unconjugated bilirubin that were not different between Ang II + UGT1A1 AS– and Ang II + apocynin + UGT1A1 AS–treated mice (Table 1). UGT1A1 AS treatment also resulted in a similar decrease in the level of conjugated bilirubin in both Ang II + UGT1A1 AS– and Ang II + apocynin+ UGT1A1 AS–treated mice (Table 1).
Table 1.
Plasma total bilirubin, direct (conjugated) bilirubin, and unconjugated bilirubin levels
| Group | Total bilirubin, mg/dl | Direct bilirubin, mg/dl | Unconjugated bilirubin, mg/dl |
|---|---|---|---|
| Ang II | 1.32±0.06 | 0.13±0.02 | 1.18±0.03 |
| Ang II + Apo | 1.30±0.09 | 0.13±0.01 | 1.30±0.09 |
| Ang II + UGT1A1 AS | 1.92±0.1 * | 0.04±0.01* | 1.88±0.1* |
| Ang II + Apo + UGT1A1 AS | 1.97±0.12* | 0.02±0.01* | 1.95±0.13* |
Plasma was collected at the end of the experimental protocol. Total and direct bilirubin levels were measured independently and use to determine the level of unconjugated bilirubin. n = 5 per group.Abbreciations: Ang II, angiotensin II; Apo, apocynin treated; AS, antisense; UDP-glucuronosyltransferase 1A1.*P < 0.05 compared with Ang II treated.
Blockade of NADPH oxidase does not affect the blood pressure response to moderate hyperbilirubinemia (MHyB) in Ang II–dependent hypertension
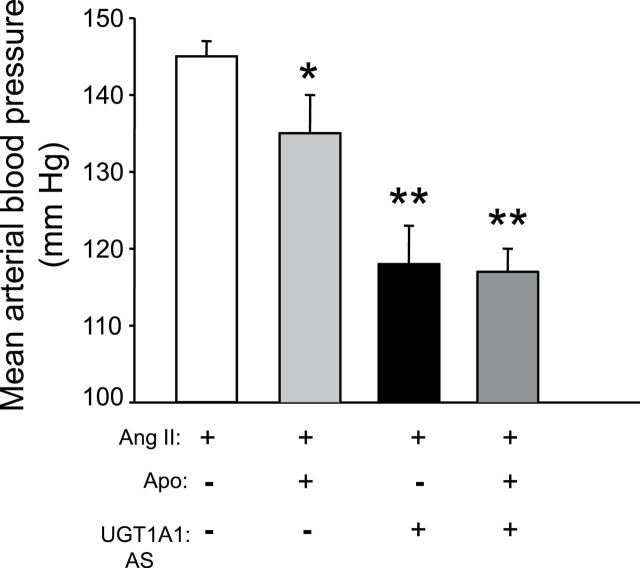
To determine the role of superoxide anion production to the antihypertensive actions of MHyB in Ang II–dependent hypertension, we administered the NADPH oxidase inhibitor apocynin to block superoxide production before the development of moderate hyperbilirubinemia. We then determined the ability of MHyB to decrease blood pressure in the setting of decreased NAD(PH-mediated superoxide production. Apocynin administration by itself decreased blood pressure in Ang II–infused mice from 145±2mm Hg to 135±4mm Hg (Figure 1). MHyB by itself lowered blood pressure to 118±5mm Hg in Ang II–infused mice (Figure 1). Prior administration of apocynin had no effect on the blood pressure response to MHyB in Ang II–infused mice with blood pressure averaging 116±2mm Hg in apocynin + UGT1A1 AS–treated mice (Figure 1).
Figure 1.
Effects of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase inhibition with apocynin on blood pressure response to moderate hyperbilirubinemia (MHyB). Mice were treated with apocynin (Apo, 14mM) in drinking water 2 days before initiation of MHyB. MHyB was induced by treatment with UDP-glucuronosyltransferase 1A1 (UGT1A1) antisense (AS) morpholinos (16mg/kg) every third day throughout the protocol. Ang II (1mg/kg/min) was delivered by osmotic minipump starting at day 6 after apocynin treatment and continued for 7 days. n = 6 per group. * P < 0.05 as compared with Ang II. ** P < 0.05 as compared with Ang II + Apo treated.
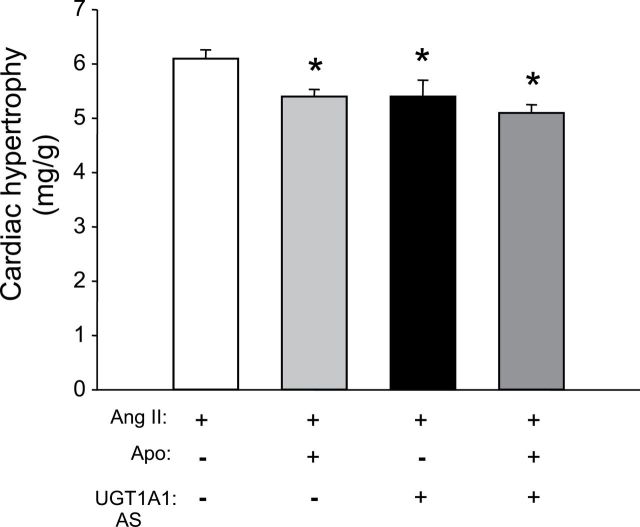
MHyB and NADPH oxidase inhibition decrease cardiac hypertrophy in Ang II–treated mice
The effects of MHyB and NADPH oxidase inhibition alone or together on cardiac hypertrophy in Ang II–treated mice were determine at the end of the study. Cardiac hypertrophy measured as the ratio of heart weight to body weight averaged 6.1 + 0.16mg/g in mice infused with Ang II (Figure 2). The level of cardiac hypertrophy was significant decreased by either apocynin or MHyB treatment alone or by a combination of the 2 averaging 5.4 + 0.1 vs. 5.4 + 0.3 vs. 5.1 + 0.15 in each group, respectively (Figure 2).
Figure 2.
Cardiac hypertrophy as determined by heart weight to body weight ratio. Body and heart weights were obtained at the end of the experimental protocol. n = 6 per group. Apo, apocynin; AS, antisense; UGT1A1, UDP-glucuronosyltransferase 1A1. * P < 0.05 as compared with angiotensin II (Ang II).
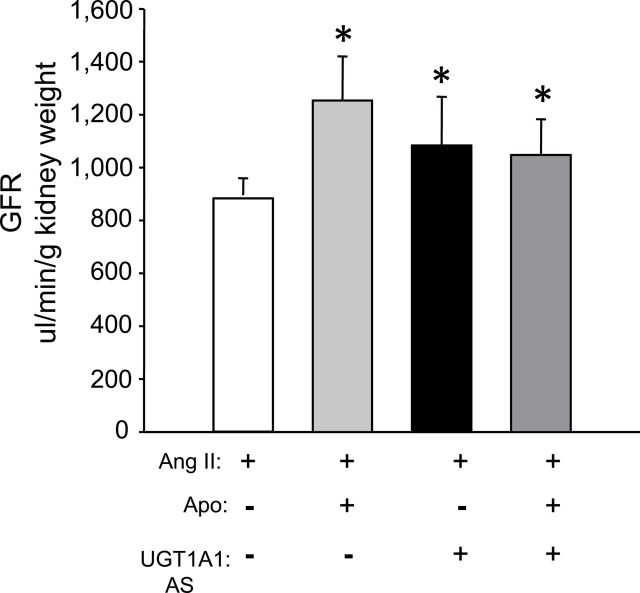
Blockade of NADPH oxidase and moderate hyperbilirubinemia increase GFR in Ang II–dependent hypertension
The effects of NADPH oxidase inhibiton and MHyB by themselves or in combination on GFR was determined in Ang II–infused mice. GFR was measured by continuous infusion of FITC-labeled insulin on days 5 and 6 after implantation of the Ang II osmotic minipumps. GFR averaged 883±112 μl/min/gKW in mice receiving Ang II infusion. Blockade of NADPH oxidase with apocynin as well as MHyB resulted in significant increases in GFR, which averaged 1254±167 and 983±125 μl/min/gKW in each group, respectively (Figure 3). Combination of apocynin with MHyB did not result a further increase in GFR, which averaged 1050±136 μl/min/gKW in this group (Figure 3).
Figure 3.
Glomerular filtration rate (GFR) in angiotensin II (Ang II)–, Ang II + apocynin (Apo) –, Ang II + UDP-glucuronosyltransferase 1A1 (UGT1A1) antisense (AS)–, and Ang II + Apo + UGT1A1 AS–treated mice. GFR was measured on days 8 and 9 after Ang II administration by steady-state infusion of fluorescein isothiocyanate (FITC)-labeled inulin (10.5mg/min). Plasma samples (50ml) were obtained by retro-orbital bleeding. Levels of FITC-labeled inulin in plasma were measured from 5ml of plasma by a fluorescence based assay. n = 4 per group. * P < 0.05 as compared with Ang II.
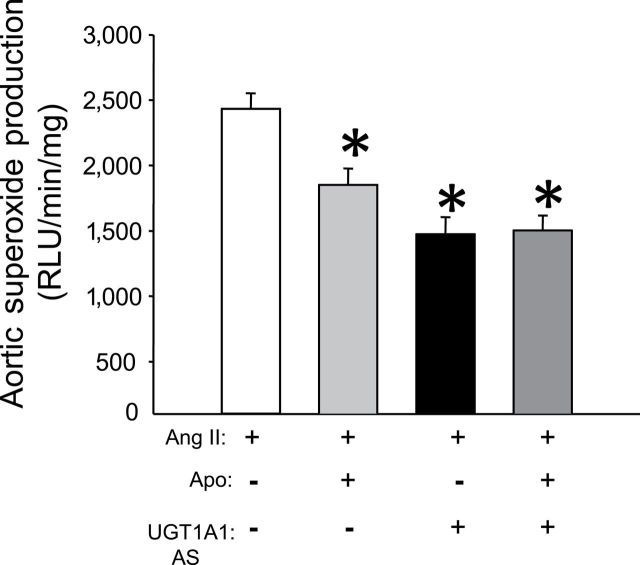
Both NADPH oxidase inhibition with apocynin and moderate hyperbiliriubinemia decrease vascular Ang II–dependent superoxide production
To determine the extent of superoxide inhibition achieved with apocynin as well as MHyB in this study, superoxide production was measured in isolated segments of the aorta. Aortic superoxide production averaged 2433±120 RLU/s in Ang II–infused mice (Figure 4). Apocynin treatment resulted in a significant decrease in aortic superoxide production to 1851±125 RLU/s (Figure 4). MHyB also resulted in a significant decrease in aortic superoxide production to 1473±132 RLU/s. Combination of apocynin and MHyB resulted in similar decrease in aortic superoxide production as either agent alone, averaging 1503±115 RLU/s (Figure 4).
Figure 4.
Aortic superoxide production in angiotension II (Ang II)–, Ang II + apocinin (Apo)–, Ang II + UDP-glucuronosyltransferase 1A1 (UGT1A1) antisense (AS)–, Ang II + Apo + UGT1A1 AS–treated mice. Superoxide levels were measured from aortic lysates by the lucigenin chemiluminescence method. Samples were obtained from mice at the end of the experimental protocol. n = 4 per group. RLU, relative light units. * P < 0.05 as compared with Ang II.
DISCUSSION
Previous studies from our laboratory have demonstrated that moderate increases in plasma bilirubin levels (approximately 2-fold) can attenuate the development of Ang II–dependent hypertension and also improve renal blood flow and GFR.5,6 These effects were also associated with a decrease in Ang II–mediated vascular superoxide production and an increase in the bioavailability of nitric oxide.5 This study was designed to test importance of blockade of superoxide anion production with the NADPH oxidase inhibitor apocynin to the antihypertensive actions of MHyB. Blockade of superoxide production alone resulted in a modest 10mm Hg decrease in blood pressure, which is similar to that in previous studies that have demonstrated that blockade of NADPH oxidase with apocynin can attenuate Ang II–dependent and other forms of hypertension.18–20 However, several studies have also reported no effect of blockade of NADPH oxidase on blood pressure using similar doses of apocynin as those used in this study.13,21 MHyB resulted in a near normalization of blood pressure in Ang II–infused mice, and prior blockade of NADPH oxidase with apocynin had no effect on the blood pressure response to MHyB in this model. This would suggest that the ability of MHyB to decrease Ang II–mediated superoxide production contributes only to a portion of the antihypertensive action of MHyB.
One possible interpretation of this data is that MHyB was more effective at inhibiting NADPH oxidase activity than the dose of apocynin used in this study. However, there are 2 factors that argue against this point. First, the dose of apocynin used in this study was based on previous studies and is higher than doses previously described in studies that have administered apocynin by oral gavage, food, or drinking water.20,22,23 Second, the results from the vascular measurement of NADPH oxidase activity which demonstrated equal blockade obtained with each compound. Both MHyB and apocynin treatment were equally effective in decreasing Ang II–mediated superoxide production, and no further decrease in Ang II–mediated superoxide production was observed in MHyB mice pretreated with apocynin. Although NADPH oxidase is the major source of superoxide anion in Ang II–treated mice, it is possible that other forms of reactive oxygen species may play a critical role in the development of hypertension in this model. Potential sources of reactive oxygen species generation include xanthine oxidase, cyclooxygenase, lipoxygenase, mitochondrial electron transport enzymes, hydrogen peroxide generation, and uncoupled nitric oxide synthase. It is possible that MHyB can act to attenuate reactive oxygen species generation from these other sources to result in further decreases in blood pressure. For example, heme oxygenase 1 induction, which results in increased production of bilirubin, has been reported to increase endothelial nitric oxide synthase (eNOS) levels and decrease the levels of inducible nitric oxide synthase (iNOS) in the vasculature.24,25 Studies in both bilirubin-infused rats and cultured murine heart endothelial immortalized cells have also demonstrated that increases in unconjugated bilirubin alone can result in decreases in the levels of iNOS.10,26 iNOS can generate superoxide anion on its own, and in the setting of increase NADPH oxidase activity, the nitric oxide generated by iNOS can result in the formation of peroxynitrite, which is a potent reactive oxygen species.27 Heme oxygenase 1 induction has also been demonstrated to increase the levels of important antioxidant proteins, superoxide dismutase, and catalase in the vasculature and the kidney.17,28 However, the specific role of bilirubin generation in this response is not known.
The results of this study demonstrate that blockade of superoxide production only accounts for a portion of the antihypertensive actions of MHyB. This raises the question of by what other mechanisms does MHyB lower blood pressure. We have previously demonstrated that MHyB was associated with a significant increase in the bioavailability of nitric oxide in Ang II hypertensive mice.5 This increase in nitric oxide levels could result in alterations in vascular resistance and contribute to the antihypertensive actions of MHyB. It is also possible the increases in plasma bilirubin may have effects to alter vascular resistance independent of nitric oxide. For example, bilirubin may alter ion channels in the vasculature to promote vasorelaxation by inhibition of calcium entry or stimulation of potassium channels. These possibilities need to be more carefully examined in future studies.
It is interesting to note that both NADPH blockade with apocynin and MHyB resulted in an increase in GFR in Ang II–infused mice. This effect was similar to the effect of these treatments on vascular superoxide production. The combination of these treatments did not result in further improvements of GFR over improvements observed by treatment with either agent alone. This suggests that decrease in GFR in Ang II–infused mice may be mediated by increased renal vascular superoxide production and not through other reactive oxygen species generating pathways. Although NADPH blockade and MHyB both resulted in similar effects on GFR, MHyB resulted in a greater decrease in blood pressure. Although the exact mechanism responsible for this difference was not determined in this study, these results suggest that MHyB may have additional effects to increase renal tubular sodium excretion compared with blockade of NADPH oxidase alone. This would result in a greater natriuresis and a further leftward shift of the pressure–natriuresis curve in MHyB mice, resulting in a greater drop in blood pressure. Induction of heme oxygenase 1 and subsequent increases in bilirubin generation have been associated with decreases in sodium reabsorption, whereas blockade of heme oxygenase 1 and decreases in bilirubin generation have been associated with increased sodium reabsorption and a rightward shift in the pressure–natriuresis response.29,30 Studies in cultured mouse thick ascending loop of Henle cells have also demonstrated that blockade of bilirubin formation is associated with increased Ang II–mediated sodium reabsorption.31 Whether moderate increases in plasma bilirubin can have similar effects as direct changes in renal bilirubin generation is not known and needs to be specifically addressed in future studies.
Similar to the observed changes in GFR in this study, both NADPH blockade with apocynin and MHyB were able to attenuate Ang II–induced cardiac hypertrophy in this study. Previous studies have demonstrated a critical role for Ang II–mediated superoxide production in the development of cardiac hypertrophy.32,33 Although blockade of NADPH oxidase with apocynin treatment did not result in as great a decrease in blood pressure as MHyB, it was equally as effective in attenuating Ang II–induced cardiac hypertrophy. Combination of NADPH oxidase inhibition and MHyB had a slight but not statistically significant effect to further decrease cardiac hypertrophy.
In summary, our results demonstrate that blockade of superoxide production by moderate increases in plasma bilirubin only accounts for a portion of the antihypertensive effects observed in Ang II–dependent hypertension. It is possible that other antioxidant properties of bilirubin account for the remainder of the blood pressure lowering effects in Ang II hypertension. Although moderate hyperbilirubinemia improves renal hemodynamics, it may also have effects on renal tubule cells to decrease sodium reabsorption. Whether these effects are also mediated by an antioxidant mechanism or a novel mechanism that blunts renal sodium reabsorption is not known at this time. These results add further support for the potential of hepatic targeting of UGT1A1 to increase unconjugated bilirubin levels as a potential antihypertensive therapy.
DISCLOSURE
The authors declared no conflict of interest.
ACKNOWLEDGMENT
The authors would like to acknowledge the support of grants from the National Heart, Lung and Blood Institute (PO1HL-51971, HL088421 to D.E.S.).
REFERENCES
- 1. Hopkins PN, Wu LL, Hunt SC, James BC, Vincent GM, Williams RR. Higher serum bilirubin is associated with decreased risk for early familial coronary artery disease. Arterioscler Thromb Vasc Biol 1996; 16: 250–255 [DOI] [PubMed] [Google Scholar]
- 2. Perlstein TS, Pande RL, Creager MA, Weuve J, Beckman JA. Serum total bilirubin level, prevalent stroke, and stroke outcomes: NHANES 1999–2004. Am J Med 2008; 121: 781–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Inoguchi T, Sasaki S, Kobayashi K, Takayanagi R, Yamada T. Relationship between Gilbert syndrome and prevalence of vascular complications in patients with diabetes. JAMA 2007; 298: 1398–1400 [DOI] [PubMed] [Google Scholar]
- 4. Lin JP, O’Donnell CJ, Schwaiger JP, Cupples LA, Lingenhel A, Hunt SC, Yang S, Kronenberg F. Association between the UGT1A1*28 allele, bilirubin levels, and coronary heart disease in the Framingham Heart Study. Circulation 2006; 114: 1476–1481 [DOI] [PubMed] [Google Scholar]
- 5. Vera T, Granger JP, Stec DE. Inhibition of bilirubin metabolism induces moderate hyperbilirubinemia and attenuates ANG II-dependent hypertension in mice. Am J Physiol Regul Integr Comp Physiol 2009; 297: R738–R743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vera T, Stec DE. Moderate hyperbilirubinemia improves renal hemodynamics in ANG II-dependent hypertension. Am J Physiol Regul Integr Comp Physiol 2010; 299: R1044–R1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stocker R. Antioxidant activities of bile pigments. Antioxid Redox Signal 2004; 6: 841–849 [DOI] [PubMed] [Google Scholar]
- 8. Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science 1987; 235: 1043–1046 [DOI] [PubMed] [Google Scholar]
- 9. Bulmer AC, Blanchfield JT, Toth I, Fassett RG, Coombes JS. Improved resistance to serum oxidation in Gilbert’s syndrome: a mechanism for cardiovascular protection. Atherosclerosis 2008; 199: 390–396 [DOI] [PubMed] [Google Scholar]
- 10. Lanone S, Bloc S, Foresti R, Almolki A, Taille C, Callebert J, Conti M, Goven D, Aubier M, Dureuil B, El Benna J, Motterlini R, Boczkowski J. Bilirubin decreases nos2 expression via inhibition of NADPH oxidase: implications for protection against endotoxic shock in rats. FASEB J 2005; 19: 1890–1892 [DOI] [PubMed] [Google Scholar]
- 11. Modlinger P, Chabrashvili T, Gill PS, Mendonca M, Harrison DG, Griendling KK, Li M, Raggio J, Wellstein A, Chen Y, Welch WJ, Wilcox CS. RNA silencing in vivo reveals role of p22phox in rat angiotensin slow pressor response. Hypertension 2006; 47: 238–244 [DOI] [PubMed] [Google Scholar]
- 12. Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest 1996; 97: 1916–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu F, Wei CC, Wu SJ, Chenier I, Zhang SL, Filep JG, Ingelfinger JR, Chan JS. Apocynin attenuates tubular apoptosis and tubulointerstitial fibrosis in transgenic mice independent of hypertension. Kidney Int 2009; 75: 156–166 [DOI] [PubMed] [Google Scholar]
- 14. Susztak K, Raff AC, Schiffer M, Bottinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes 2006; 55: 225–233 [PubMed] [Google Scholar]
- 15. Vera T, Taylor M, Bohman Q, Flasch A, Roman RJ, Stec DE. Fenofibrate prevents the development of angiotensin II-dependent hypertension in mice. Hypertension 2005; 45: 730–735 [DOI] [PubMed] [Google Scholar]
- 16. Csongradi E, Storm MV, Stec DE. Renal inhibition of heme oxygenase-1 increases blood rressure in angiotensin II–dependent hypertension. Int J Hypertens 2012; 2012: 497213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vera T, Kelsen S, Yanes LL, Reckelhoff JF, Stec DE. HO-1 induction lowers blood pressure and superoxide production in the renal medulla of angiotensin II hypertensive mice. Am J Physiol Regul Integr Comp Physiol 2007; 292: R1472–R1478 [DOI] [PubMed] [Google Scholar]
- 18. Hu L, Zhang Y, Lim PS, Miao Y, Tan C, McKenzie KU, Schyvens CG, Whitworth JA. Apocynin but not L-arginine prevents and reverses dexamethasone-induced hypertension in the rat. Am J Hypertens 2006; 19: 413–418 [DOI] [PubMed] [Google Scholar]
- 19. Tain YL, Hsu CN, Huang LT, Lau YT. Apocynin attenuates oxidative stress and hypertension in young spontaneously hypertensive rats independent of ADMA/NO pathway. Free Radic Res 2012; 46: 68–76 [DOI] [PubMed] [Google Scholar]
- 20. Virdis A, Neves MF, Amiri F, Touyz RM, Schiffrin EL. Role of NADPH oxidase on vascular alterations in angiotensin II-infused mice. J Hypertens 2004; 22: 535–542 [DOI] [PubMed] [Google Scholar]
- 21. Kopkan L, Huskova Z, Vanourkova Z, Thumova M, Skaroupkova P, Maly J, Kramer HJ, Dvorak P, Cervenka L. Reduction of oxidative stress does not attenuate the development of angiotensin II-dependent hypertension in Ren-2 transgenic rats. Vascul Pharmacol 2009; 51: 175–181 [DOI] [PubMed] [Google Scholar]
- 22. Jia Z, Guo X, Zhang H, Wang MH, Dong Z, Yang T. Microsomal prostaglandin synthase-1-derived prostaglandin E2 protects against angiotensin II-induced hypertension via inhibition of oxidative stress. Hypertension 2008; 52: 952–959 [DOI] [PubMed] [Google Scholar]
- 23. Kopkan L, Hess A, Huskova Z, Cervenka L, Navar LG, Majid DS. High-salt intake enhances superoxide activity in eNOS knockout mice leading to the development of salt sensitivity. Am J Physiol Renal Physiol 2010; 299: F656–F663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ahmad M, Turkseven S, Mingone CJ, Gupte SA, Wolin MS, Abraham NG. Heme oxygenase-1 gene expression increases vascular relaxation and decreases inducible nitric oxide synthase in diabetic rats. Cell Mol Biol (Noisy -le-grand) 2005; 51: 371–376 [PubMed] [Google Scholar]
- 25. Kawamura K, Ishikawa K, Wada Y, Kimura S, Matsumoto H, Kohro T, Itabe H, Kodama T, Maruyama Y. Bilirubin from heme oxygenase-1 attenuates vascular endothelial activation and dysfunction. Arterioscler Thromb Vasc Biol 205; 25: 155–160 [DOI] [PubMed] [Google Scholar]
- 26. Mazzone GL, Rigato I, Tiribelli C. Unconjugated bilirubin modulates nitric oxide production via iNOS regulation. Biosci Trends 2010; 4: 244–248 [PubMed] [Google Scholar]
- 27. Miller AA, Megson IL, Gray GA. Inducible nitric oxide synthase-derived superoxide contributes to hypereactivity in small mesenteric arteries from a rat model of chronic heart failure. Br J Pharmacol 2000; 131: 29–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Turkseven S, Kruger A, Mingone CJ, Kaminski P, Inaba M, Rodella LF, Ikehara S, Wolin MS, Abraham NG. Antioxidant mechanism of heme oxygenase-1 involves an increase in superoxide dismutase and catalase in experimental diabetes. Am J Physiol Heart Circ Physiol 2005; 289: H701–H707 [DOI] [PubMed] [Google Scholar]
- 29. Li N, Yi F, Dos Santos EA, Donley DK, Li PL. Role of renal medullary heme oxygenase in the regulation of pressure natriuresis and arterial blood pressure. Hypertension 2007; 49: 148–154 [DOI] [PubMed] [Google Scholar]
- 30. Rodriguez F, Kemp R, Balazy M, Nasjletti A. Effects of exogenous heme on renal function: role of heme oxygenase and cyclooxygenase. Hypertension 2003; 42: 680–684 [DOI] [PubMed] [Google Scholar]
- 31. Young SC, Storm MV, Speed JS, Kelsen S, Tiller CV, Vera T, Drummond HA, Stec DE. Inhibition of biliverdin reductase increases ANG II-dependent superoxide levels in cultured renal tubular epithelial cells. Am J Physiol Regul Integr Comp Physiol 2009; 297: R1546–R1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Byrne JA, Grieve DJ, Bendall JK, Li JM, Gove C, Lambeth JD, Cave AC, Shah AM. Contrasting roles of NADPH oxidase isoforms in pressure-overload versus angiotensin II-induced cardiac hypertrophy. Circ Res 2003; 93: 802–805 [DOI] [PubMed] [Google Scholar]
- 33. Nakagami H, Takemoto M, Liao JK. NADPH oxidase-derived superoxide anion mediates angiotensin II-induced cardiac hypertrophy. J Mol Cell Cardiol 2003; 35: 851–859 [DOI] [PubMed] [Google Scholar]