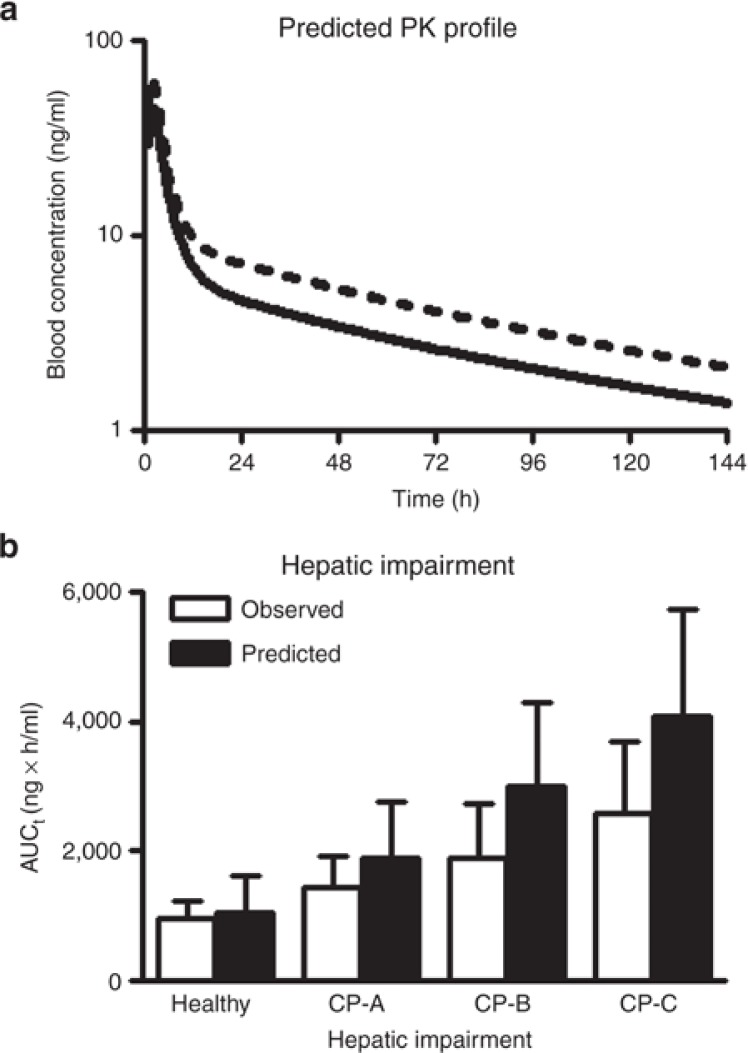
Figure 5.
Prediction of pharmacokinetic parameters in case studies, for a drug–drug interaction (a) and in patients with hepatic impairment (b) by the developed base model. (a) Sirolimus blood concentrations in a healthy adult after an oral administration of 10 mg of sirolimus alone (solid line) or with coadministration of 120 mg of diltiazem (dashed line). A virtual drug–drug interaction study was carried out with the model for 180 virtual healthy adults as identical as possible to those in the clinical study in terms of age and male–female ratio of subjects using Simcyp version 11 and each line shows the mean of individual data. In the study, the concentration-time profiles of sirolimus were simulated by the PBPK models (Model 1, see Table 1 and Figure 4). (b) Effect of hepatic impairment on AUC of sirolimus after a single oral administration of a 15 mg dose of sirolimus. This pharmacokinetic study was carried out for individual virtual healthy adults, and virtual patients with cirrhosis Child-Pugh grade A, B, and C (CP-A, CP-B, and CP-C, respectively) implemented in Simcyp version 11 (100 subjects for each population). Obtained AUC values from the virtual study with the PBPK model (Model 1) were compared with observed AUC values (mean ± SD) in a published study.30,40