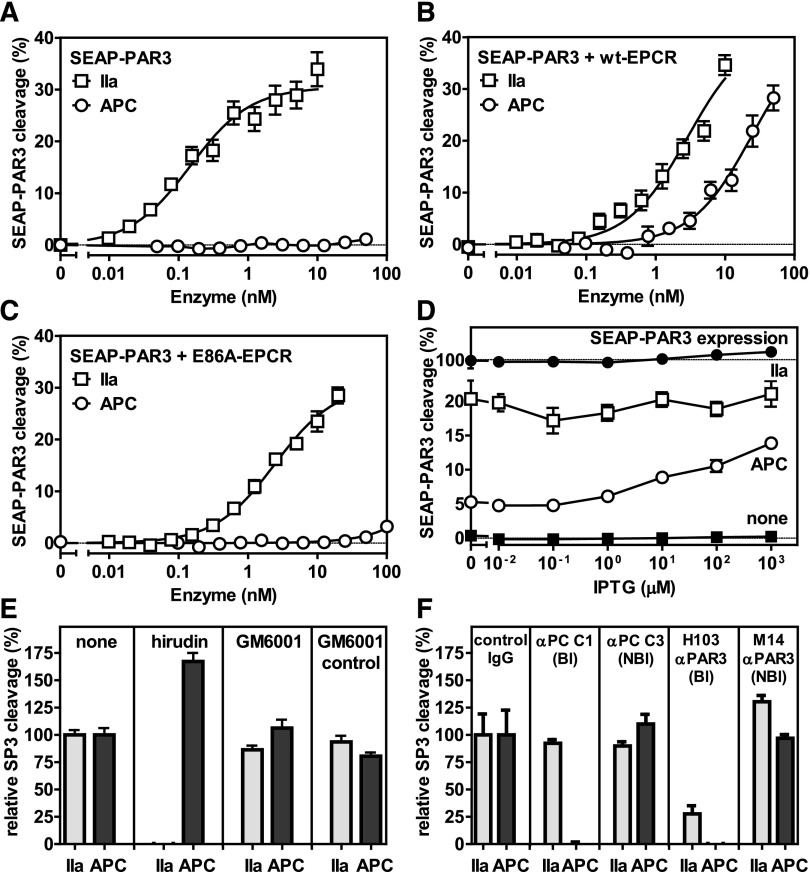
Figure 1.
EPCR-dependent PAR3 cleavage by APC. APC-mediated cleavage of PAR3 was analyzed by the proteolytic release of SEAP from a SEAP-PAR3 fusion protein expressed in HEK-293 cells in the absence or presence of stable EPCR coexpression. Dose response of PAR3 cleavage by thrombin (IIa) (□) or APC (○) in the (A) absence of EPCR and in the presence of (B) wt-EPCR or (C) E86A-EPCR defective in APC binding. (D) PAR3 cleavage by 2 nM thrombin (□) or 20 nM APC (○) in SEAP-PAR3 HEK-293 cells in the presence of isopropyl β-D-1-thiogalactopyranoside (IPTG) tunable wt-EPCR coexpression (EPCR-pTUNE). IPTG did not affect SEAP release from cells in the absence of protease (■) or the total SEAP-PAR3 expression on cells (●). PAR3 cleavage was expressed as a percentage of the total available SEAP-PAR3 on the cells. (E-F) Specificity controls of PAR3 cleavage by 10 nM thrombin or 20 nM APC in wt-EPCR/SEAP-PAR3 cells (E). Inhibitors used were directed against thrombin (20 U/mL hirudin) and matrix metalloproteinases (10 μM GM6001 or GM6001 inactive control). (F) Blocking (Bl) and nonblocking (NBl) antibodies (all 20 μg/mL) were directed against APC or PAR3. PAR3 cleavage was expressed relative to the cleavage in the absence of inhibitors (E) or in the presence of nonimmune IgG antibodies (F). Data points represent the mean ± SEM (n ≥ 3). Single amino acid abbreviations denote A, Ala; and E, Glu.