Fig. 1.
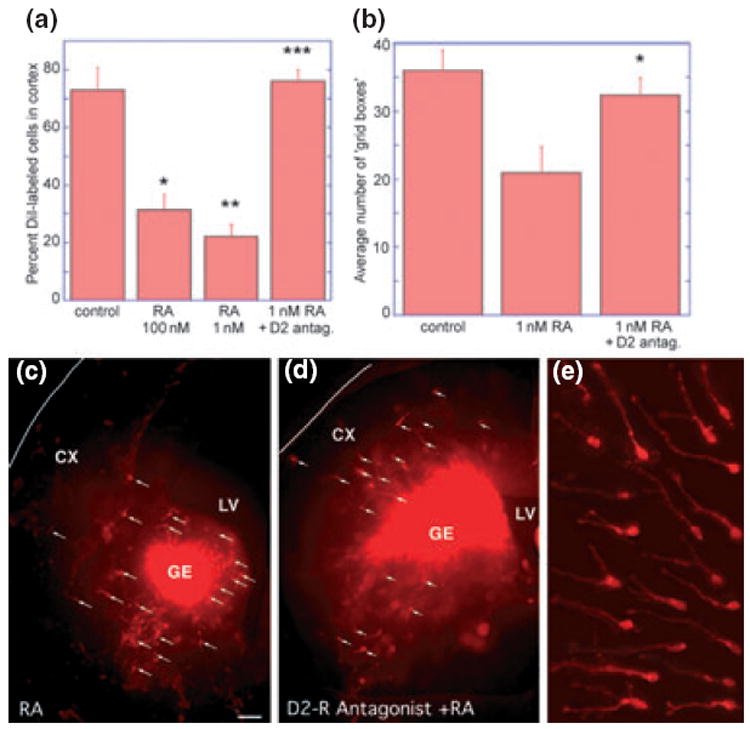
Retinoic acid reduces the number of cells migrating from the ganglionic eminence (GE) to the cortex in slice cultures, and this reduction can be reversed by addition of a D2 receptor antagonist. (a) Addition of all-trans RA (100 or 1 nM) significantly reduced the percentage of DiI-labelled cells migrating to the cortex. This reduction could be reversed, if the D2-dopamine receptor was blocked with a specific antagonist (20 μM eticlopride). (*100 nM RA vs. control t = 4.43, p = 0.003, **1 nM RA vs. control t = 6.40, p = 0.0001, ***1 nM RA vs. 1 nM RA + eticlopride t = 7.80, p < 0.0001. (b) Similarly, migration indicated by the average number of grid boxes that contained DiI-labelled cells was reduced, following 1 nM RA treatment, but approached control levels by the addition of the D2-dopamine receptor antagonist eticlopride (*1 nM RA vs. 1 nM RA + eticlopride t = 2.38, p = 0.03. (c–e) Micrographs of experimental brain slices photographed at one focal plane. E15 forebrain slices were labelled with identical amounts of DiI crystals placed in the ganglionic eminence (GE); Panels (c) and (d) are overviews, and panel (e) shows assorted migrating precursors with axonal processes and growth cones heading to the left and up. Far fewer DiI-labelled cells with processes (white arrows) can be seen in the cerebral wall (CX) after 2 days of all-trans RA exposure (c), as compared with the slice exposed to RA and the dopamine D2-receptor antagonist eticlopride (d). LV, lateral ventricle.
