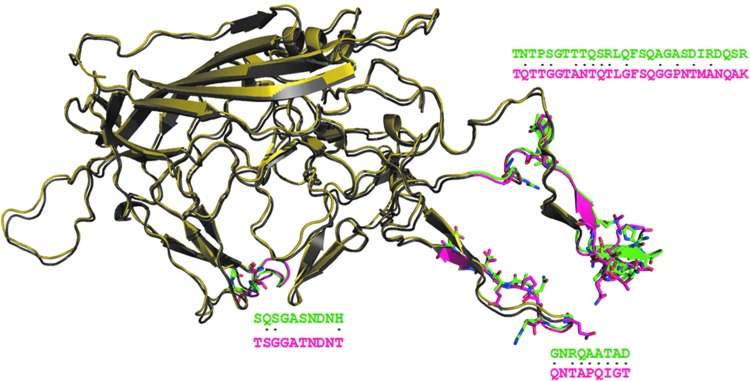
FIG. 7.
Comparison of AAV8 and AAV2 capsid structures. Superimposition of AAV2 (colored yellow) and AAV8 (colored gray) capsid structures are shown. The green-colored region in AAV2 and the magenta-colored region in AAV8 show drastic residue substitutions. The residues that are drastically substituted are represented as sticks and are also marked as dots (.) in the sequence alignment between AAV2 and AAV8. These regions with drastic substitutions are away from the phosphodegron-containing and receptor-binding regions. It can be speculated that these regions could interact with the N-terminal region of the capsid structure for which the crystal structure is currently not available. Drastic differences in the interface could have an influence on the interaction with the N-terminal region of capsid structure flanking the K137. This could affect the conformation of region spanning K137 in AAV2 and AAV8 and thus contribute to their varied transduction efficiency as seen experimentally with the K137R mutation in AAV2 and AAV8 serotypes (Gabriel et al., 2013). Color images available online at www.liebertpub.com/hgtb