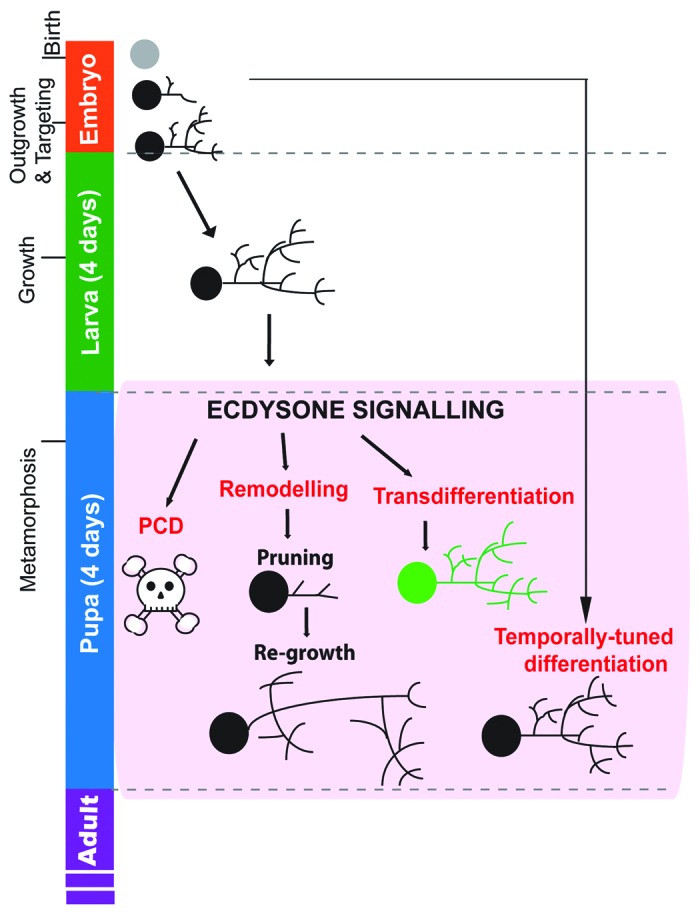
Figure 1. Major forms of neuronal remodeling during ecdysone-driven metamorphosis in Drosophila. Developmental stages of Drosophila melanogaster are shown (at 25°C). During embryonic development (24 h), the embryonic neurons are born and their axons and dendrites subsequently grow to their targets and connect into appropriate circuits for function during larval stages. Most differentiate at this time into mature functional neurons. During larval stages, most neurons undergo expansional growth to accommodate the increasing animal size. Throughout larval stages, subsets of post-embryonic neuroblast lineages (not shown in figure) undergo division to produce new postmitotic neurons. This peaks during the final 48 h of larval life. Ecdysone signaling increases prior to pupariation and prompts metamorphosis (in combination with a drop in juvenile hormone titer and the attainment of critical weight). Ecdysone titers fluctuate throughout metamorphosis (not shown here). Ecdysone signaling prompts 4 major forms of neuronal remodeling that occurs during metamorphosis in the pupa; Programmed cell death (PCD), structural remodeling, transdifferentiation or temporally-tuned differentiation. The exact time of remodeling differs between neuronal subsets. Programmed cell death kills specific neuronal subsets with high segmental and subtype specificity. Structural remodeling is neuronal subtype-specific and remodels larval arbors into adult-specific circuits. Certain neurons remodel only axons, or only dendrites, or both. The first step is pruning of branches back to proximal projections, followed by re-targeting, often to distinct targets. Transdifferentiation is rare and involves the changing of effector gene expression so that the neurons adopt a novel function. Temporally-tuned differentiation has been observed for numerous neuronal populations, involving the developmental freezing of neurons at earlier stages of development prior to mature effector gene expression and axo-dendritic arbor outgrowth.
