Figure S3.
Vpu Interactions, Topology, and Activity in Proteoliposomes, Related to Figure 3
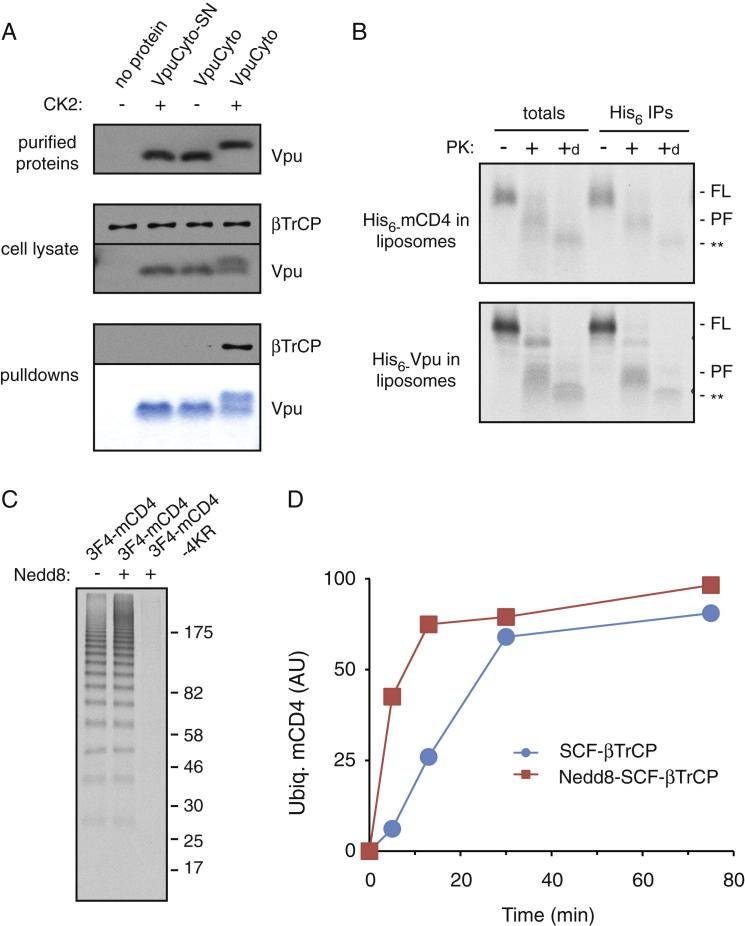
(A) His6-tagged cytosolic domains of Vpu or Vpu-SN were expressed and purified from E. coli and incubated with CK2 as indicated. An aliquot was analyzed by immunoblotting (top) to verify efficient phosphorylation (as evidenced by a shift on the gel). The purified proteins were incubated with HeLa cytosol containing overexpressed βTrCP1 and subjected to pulldown using immobilized Co2+. The cell lysate (containing the added purified proteins) and pulldowns were analyzed by SDS-PAGE and immunoblotting or Coomassie staining. βTrCP is selectively pulled down in a phosphor-Vpu-dependent manner.
(B) Liposomes were reconstituted with radiolabeled His6-tagged mCD4 or Vpu. They were either left untreated, digested with proteinase K (PK), or PK in the presence of 0.5% Triton X-100 (indicated by subscript ‘d’). The samples were either analyzed directly, or immunoprecipitated using an antibody against the His6 tag. The positions of full-length (FL) and primary protease-protected N-terminal fragments (PF) are indicated. ‘∗∗’ indicates a protease-resistant fragment. Note that the heterogeneous bands seen with PK digestion probably arise from variations in precisely where within the cytosolic tails PK cuts.
(C) Radiolabeled mCD4 or mCD4-4KR (in which the four lysines in the cytosolic tail of CD4 are mutated to arginines) isolated from in vitro translation reactions was coreconstituted with recombinant Vpu into liposomes. The resuspended liposome sample was subjected to ubiquitination reactions using purified SCFβTrCP that had or had not been modified with Nedd8 as indicated. The ubiquitinated products (isolated by pulldown via the tagged ubiquitin) of the 30 min reaction are shown.
(D) Time course of ubiquitination reactions as in C quantified by phosphorimaging shows that Neddylation of SCF improves its reaction speed.