Figure S4.
Minimal Substrate Discrimination Is Observed in the Reconstituted System, Related to Figure 4
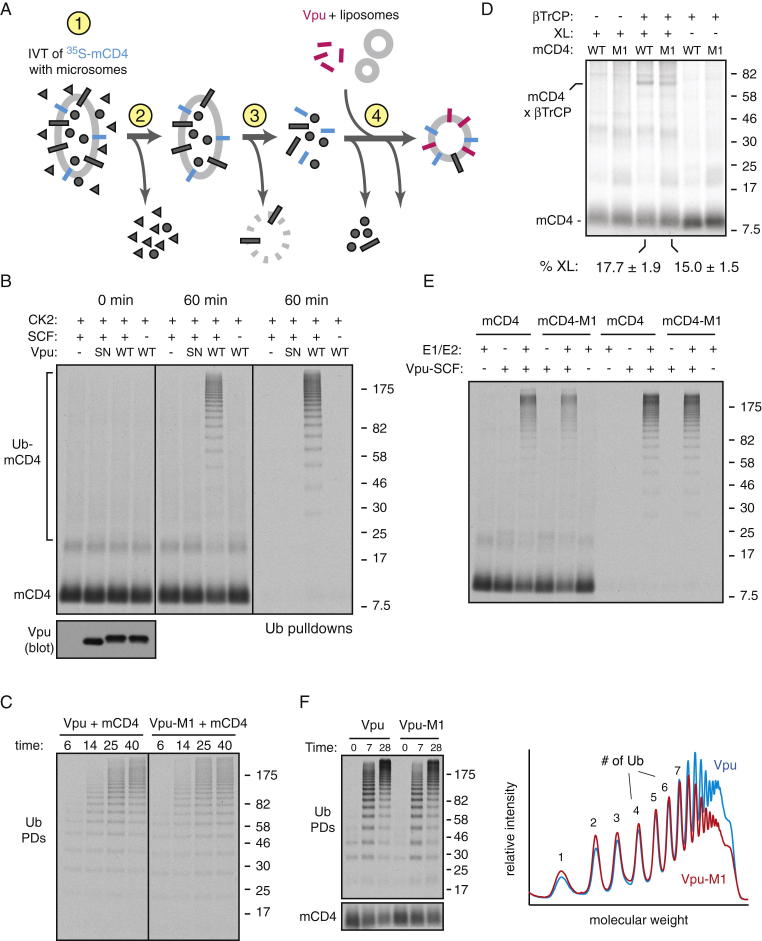
(A) Schematic depicting the procedure for preparing proteoliposomes containing radiolabeled mCD4 and recombinant Vpu. The starting in vitro translation reaction used to produce 35S-methionine-labeled mCD4 contains cytosolic proteins and microsomal proteins. These are progressively removed during the reconstitution procedure such that cytosolic proteins are not detectable in the final proteoliposomes and microsomal proteins are removed to more than 95%.
(B) Radiolabeled in vitro produced mCD4 was coreconstituted into liposomes using the procedure in A with either nothing else, recombinant Vpu, or Vpu-SN. The proteoliposomes were then treated with CK2 to phosphorylate Vpu, and subjected to ubiquitination reactions containing or lacking SCFβTrCP as indicated. The samples were analyzed by autoradiography to detect mCD4 or immunoblotting to detect Vpu. An aliquot of the samples were also subjected to pulldowns via the His6-tag on ubiquitin to visualize the ubiquitinated products. Note that ubiquitinated products (representing approximately 60% of mCD4) were only seen when the reaction contained both phospho-Vpu and SCFβTrCP. This indicates that no other ligase activity capable of ubiquitinating mCD4 is present in this reconstituted system.
(C) Radiolabeled mCD4 was produced as in A, except an additional Co+2 affinity step was included to further purify the substrate prior to reconstitution into proteoliposomes with Vpu or Vpu-M1. Shown are the ubiquitinated products at different times from a reaction similar to that in B.
(D) Proteoliposomes containing recombinant phosphorylated Vpu and the radiolabeled substrates mCD4 (or mCD4-M1) were produced as in B and incubated with recombinant βTrCP/Skp1 complex where indicated. The samples were treated with the cysteine-reactive crosslinker BMH as indicated, quenched with DTT, and analyzed by SDS-PAGE and autoradiography to detect mCD4 and mCD4-M1. Note that recombinant Vpu does not have cysteine residues and therefore does not participate in crosslinking. The position of the substrate crosslink to βTrCP is indicated. Crosslinking efficiencies for mCD4 and mCD4-M1 are nearly the same (mean ± SD; n = 6).
(E) An experiment similar to B was performed with mCD4 versus mCD4-M1 as the substrate. Note that ubiquitination is more than 50% efficient, completely dependent on the Vpu-SCF complex, and very similar between mCD4 and mCD4-M1.
(F) Radiolabeled mCD4 coreconstituted with either Vpu or Vpu-M1 in liposomes was subjected to ubiquitination. Aliquots at different time points were analyzed by ubiquitin pulldowns (top) or directly for mCD4 levels (bottom). The traces at the right are densitometry profiles of the two reactions at 7 min, illustrating a selective reduction of highly poly-ubiquitinated products selective with Vpu-M1.