Abstract
Aim:
This study investigated the immunomodulatory and anti-tumor effects of Nigella glandulifera Freyn and Sint seeds (NGS) on Ehrlich ascites carcinoma in a mouse model.
Materials and Methods:
Kunming mice with transplanted Ehrlich ascites tumor cells (EAC) were treated with NGS by oral administration. On the 11th day after the EAC implant, mouse thymus, liver, spleen and kidney tumors were removed for histopathological analysis. Blood samples were taken for hematological and biochemical analyses.
Results:
The results indicate that NGS treatment leads to an increase in TNF-α, IL-1β, and IL-2 blood serum levels. Absence of viable EAC and presence of necrotic cells were observed in the tumor tissue of the NGS-treated animals.
Conclusions:
The study results indicated that a water extract of NGS had the highest anti-tumor effect. Moreover, NGS treatment also showed an increase in the immune system activity.
Keywords: Anti-tumor, Immunomodulatory, Seeds of Nigella glandulifera Freyn
INTRODUCTION
Nigella glandulifera Freyn and Sint seeds (NGS), a traditional Uyghur medicine, has shown anti-tumor properties in Uyghur people. This study attempts to confirm these effects in vivo. The genus Nigella (Ranunculaceae) comprises of about 20 species. The seeds of N. glandulifera are commonly added to many Uyghur food preparations and NGS are reported to possess diuretic, analgesic, spasmolytic, galactagogue, and bronchodilator effects. They have been used in the treatment of tumor, edema, urinary calculus, and bronchial asthma[1,2] They also aid in improving the blood circulation as well as brain and kidney functions. N. glandulifera is mainly produced in the Xinjiang Uyghur Autonomous Region and the plant has been included in each edition of the Pharmacopoeia of the People's Republic of China since 1977. Previous studies have shown that N. glandulifera contains many chemical components including damascenine, thymoquinone, niqullon, quercetin, and saponins.
On the basis of its traditional use in tumor treatment, we deemed it appropriate to test the effects of NGS on the development of tumors in an animal model. In this study, mice with exogenously implanted Ehrlich ascites tumor cells (EAC) were treated with NGS water extracts (NGSWE) or NGS ethanol extracts (NGSEE). Subsequent tumor growth was assessed. Since immunity plays a major role in protection against cancer,[3,4,5,6] the EAC-implanted mouse models were examined to determine the effect of NGS extracts on three immunity markers: Tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and IL-2. The effects of the NGS treatments were compared to those of cyclophosphamide (CY), a standard comparator for EAC.
MATERIALS AND METHODS
Chemicals and reagents
The N. glandulifera seeds were provided by the Xinjiang Uyghur National Hospital. Cyclophosphamide was purchased from Jiangsu Hengrui Medicine Co., Ltd. (Lianyungang, China) under the State Food and Drug Administration (Batch H32020857). Mouse IL-1β ELISA (Batch 56069011), Mouse IL-2 ELISA, (Batch 55708025) and Mouse TNF-α ELISA, (Batch 55674004) kits were purchased from Bender MedSystems, Vienna, Austria.
Animals and treatment
Kunming specific pathogen-free mice (4–6 weeks old, average body weight 19 ± 2 g) were provided by the Laboratory Animal Center (LAC) of Xinjiang Medical University. The mice were bred under regular laboratory conditions (i.e., room temperature, 12/12-hour light/dark cycle, and with free access to standard rodent chow and water). The Animal Care and Use Committee of LAC approved all experimental protocols.
Fifty mice were randomly divided into five groups of ten animals each: Normal group: No intervention no NGS treatment (normal saline 20 μL/kg orally daily), Model group: EAC injected, no NGS treatment (normal saline 20 μL/kg orally daily), CY group: EAC injected, daily CY injection 20 mg/kg, NGSWE group: EAC injected, oral NGSWE 2 g/kg daily and NGSEE group: EAC cells injection, oral NGSEE 2 g/kg daily. The EAC was supplied by Wuhan University.
With the exception of the normal group, 0.2 mL (1 × 107 cells/mL) of seven-day-old EAC was transplanted into the right axilla of each mouse. The transplant operation was completed in 30 mins. Treatments were started 24 h after tumor inoculation, and were administered once a day for 10 days.
Animal observation
Animals were observed and graded daily for activity, thinness, appearance of skin and hair, appetite, and irritability. Tumor size was measured daily with a ruler. Tumor size was plotted against time to determine the tumor growth rate.
Assessment of tumor weight and thymus, liver and spleen indices
Twenty-four hours after the last treatment administration on day 10, the mice were sacrificed by cervical dislocation. The thymus, spleen and liver tumors were removed and weighed. Anti-tumor activity in vivo was expressed as an inhibitory rate calculated by using [(A–B)/A] × 100%, where A and B were the mean tumor weight of the model control and experimental groups, respectively. The spleen, liver, and thymus were evaluated by using the organ index formula: Organ weight (g)/body weight (g).
Histological investigation
A portion of the tumor~kidney, thymus, spleen and liver was fixed in a 10% buffered formalin for histological investigation and the remaining tissue was used for biochemical measurements. The histological tissues were fixed in solutions of ethanol: 70% for 3 h, 80% for 2 h, 90% for 1.5 h, 95% for 2 h, and 100% for 1 h. Subsequently, the tissues were embedded in paraffin and at least four cross-sections were taken from each tumor in the kidney, thymus, spleen and liver. Sections were 4–5 μm thick and were stained with hematoxylin and eosin. Two xylene treatment changes (2 min each) were performed and the final tissue sections were mounted with DPX mountant. The slides were observed for histopathological changes, and microphotographs were obtained by using an Olympus Bx50 microscope system (Olympus, Tokyo, Japan).
Evaluation of cytokines
According to the tube manufacturer's protocol, blood was immediately transferred into test tubes and kept at room temperature for 30 minutes. The blood was then centrifuged at 3000 rpm for 20 minutes. This was followed by serum assays for TNF-α, IL-1β, and IL-2 using the ELISA method, according to the kit manufacturer's instructions.
Statistical procedures
SPSS Version 17.0 (IBM, Armonk, NY, USA) was used to test normality and variance homogeneity. Mean and standard deviation values were calculated, followed by single-factor variance analysis and card side examination of the results. The group results were compared by ANOVA. P < 0.05 was considered statistically significant.
RESULTS
Influence of mice daily activities
Following treatment, mouse behavior, including drinking, defecation, and urination, as well as general appearance was monitored daily. No secretions from the eyes, ears, nose, or mouth were observed.
In the NGSWE group, the mice exhibited normal activity, a good mental state and had clean fur (better than in the NGSEE group). In addition, in the NGSWE group, the Tumor growth incubation period (TP) was 3 d; however, Tumor growth speed (TS) was slower than in the NGSEE group. The NGSEE group exhibited normal activity, had a good mental state, and clean fur (better than in the model group). In the CY group, there was normal activity, a good mental state, clean fur (better than in the NGSEE group), and a TT of 4 d. The CY group had the slowest TS of all of the groups. The model group (saline treatment only) showed decreased activity, had greater tumor growth, unclean fur, and exhibited listlessness with a TT of 2 d and the fastest TS of all of the groups.
Inhibition of transplanted EAC tumor growth and effects on organ weight ratio
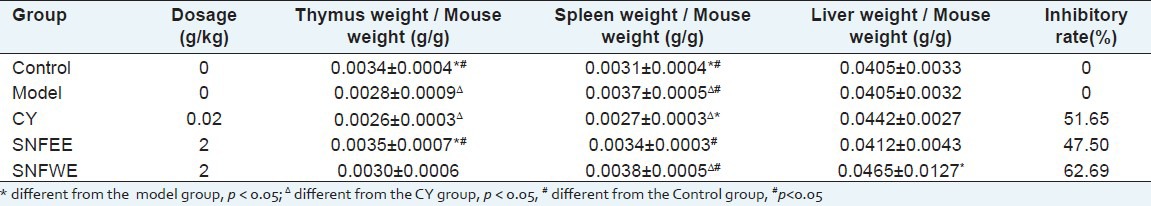
The anti-tumor effects of NGS treatment on the EAC-bearing mice are summarized in Tables 1 and 2. Treatment with CY and NGS resulted in marked suppression of tumor weight gain. Compared with the model group, the inhibitory rate for the NGSEE, NGSWE and the CY groups in the EAC-bearing mouse were 47.50%, 62.69%, and 51.65%, respectively. The NGSEE and NGSWE groups showed a significant inhibition of tumor weight gain when compared to the CY group (P < 0.05). Moreover, the spleen, liver, and thymus indices for the NGSEE and NGSWE groups did not decrease significantly compared to those in the model group [Table 2]. The results indicated that in vivo NGS treatment did not result in any significant toxic effects on the immune systems of the EAC-bearing mice.
Table 1.
Antitumor activity of the SNF in mice transplanted EAC tumor (`x±SD)
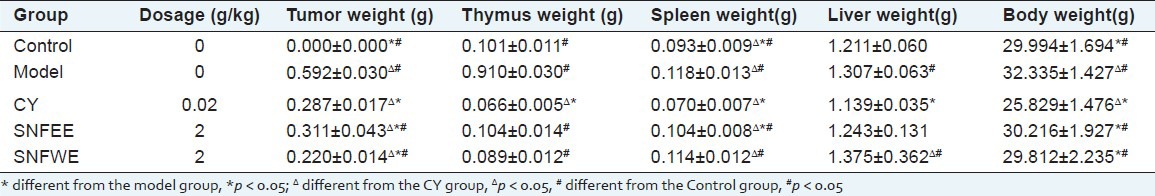
Table 2.
Inhibition of SNF on the growth of transplanted tumors in EAC induced mice and organ weight ratio (`x ± SD)
Treatment effects on IL-1β, IL-2, and TNF-α
Effects of NGS treatment on IL-1β, IL-2, and TNF-α concentrations are shown. The IL-1β, IL-2, and TNF-α concentrations were lower in the model and CY groups than in the control group. Animals treated with NGSWE and NGSEE had significantly higher TNF-α concentrations (P < 0.05) than the control groups. Compared with the model group, the NGSWE and NGSEE groups had significantly higher IL-1, IL-2 and TNF-α concentrations.
Pathological examination
Compared to the model group [Figure 1], there was more obvious tumor tissue damage in the CY group [Figure 2]. The tissue specimens from the CY group showed large areas of necrosis, mainly eosinophilic necrosis. In the NGSEE group, [Figure 3] there were small areas of tissue necrosis while in the NGSWE group [Figure 4] there were large areas of tissue necrosis. The NGSWE group tissues exhibited obvious ischemic necrosis along with phlogocyte infiltrate.
Figure 1.
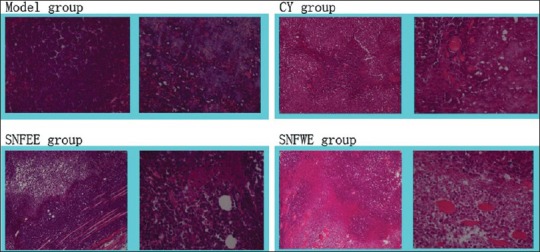
Histological section of EAC in Kunming mice
Figure 2.
The level of IL-1β; in serum
Figure 3.
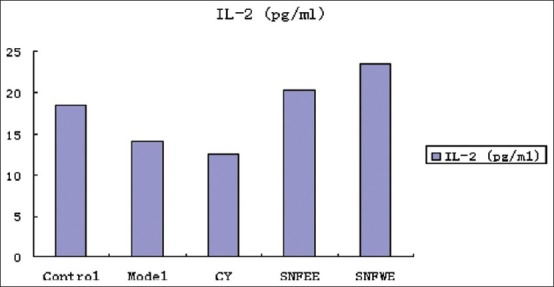
The level of IL-2 in serum
Figure 4.
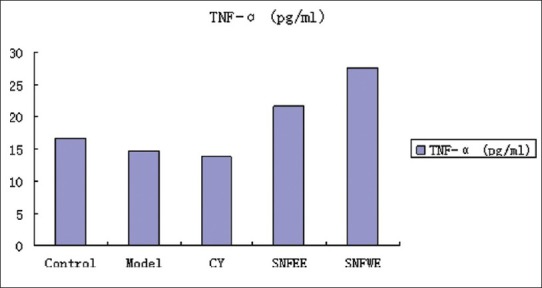
The level of TNF-α; in serum
DISCUSSION
In this study, the anti-tumor effects of NGS were evaluated in a mouse xenograft model. The results showed that NGSWE treatment had the most potent anti-tumor activity. These findings are compatible with previous reports on NGSWE treatment, which have shown that NGSWE can be applied as an anti-tumor adjuvant with an immunomodulatory effect. Thus, NGSWE has the potential to be ofgreat clinical significance.
The present study of EAC-implanted mice confirmed the anti-proliferative effects of NGS treatment on tumor cells. In addition, there was an increased spleen index following NGS treatment. The NGSWE treatment also resulted in an increase in IL-1β, IL-2, and TNF-α levels. Both IL-1β and IL-2 are important lymphokines and can promote the proliferation of T cells. In anti-tumor immunity, which is predominantly cellular, T cells are the main immune cells that can be cytotoxic directly, or indirectly through the secretion of cytokine TNF-α[7,8] Many of the cytokines, including IFN-γ and IL-2, can activate natural killer (NK) cells[9,10] In the absence of antibodies, NK cells can kill tumor cells in vivo. Activation of NK cells induces lymphokine-activated killer cells and cytotoxic T cell maturation, which in anti-tumor immunity plays an important role in regulating lymphocyte proliferation. In addition, activated NK cells can release cytokines TNF-α and IL-2 and can stimulate macrophages. TNF-α has many effects on the immune system regulation and response. It can promote blood clotting and increase the adhesion and migration capabilities of leukocytes, adjust the activation of macrophages and immune responses in tissues, and regulates the generation of blood cells and the development of lymphocytes[11,12,13,14,15] These effects and the NGSWE results indicate that NGSWE treatment can improve immune functions and inhibit tumor growth in the EAC-bearing mice. Our results show that NGSWE has inhibitory effects on neoplastic cells and the tumor inhibitory effects may contribute to immunity enhancement.
Development of anti-tumor drugs from plants is an important part of cancer research. Discovery of active ingredients and anti-tumor mechanisms is of great interest in this field of research. In this study, an anti-tumor trial involving oral NGSWE treatment showed tumor inhibitory effects. The results showed that NGSWE has an inhibitory effect higher than the required level of inhibitory efficacy in Chinese medicines (i.e., an inhibition rate of over 30%). It was observed that NGSWE treatment can reduce the tumor growth rate of the tumor-bearing mice and improve the thymus and spleen indices, indicating the presence of immunomodulatory effects.
This study confirmed that NGSWE could have an anti-tumor effect without concomitant adverse reactions. The treatment did not affect the normal behavior, growth and development of our test animals. We suggest that NGS extracts, particularly a water extract, may have the potential for future anti-tumor drug development; however, determination of NGS's active ingredient and its mechanism of action requires further studies.
ACKNOWLEDGMENTS
This research work was supported by the Xinjiang Autonomous Region Natural Science Foundation of China (2011211B27).
Footnotes
Source of Support: Xinjiang Autonomous Region Natural Science Foundation of China (2011211B27)
Conflict of Interest: None declared.
REFERENCES
- 1.Yusup A, Upur H, Baudrimont I, Umar A, Kader T, Begaud B, et al. Cytotoxicity of abnormal Savda Munziq aqueous extract in human hepatoma (hepg2) cells. Fundam Clin Pharmacol. 2005;19:465–72. doi: 10.1111/j.1472-8206.2005.00342.x. [DOI] [PubMed] [Google Scholar]
- 2.Upur H, Yusup A, Umar A, Moore N. Uighur traditional medicine syndrome of Abnormal Savda in men is associated with oxidative stress, which can be improved by Munziq and Mushil of Abnormal Savda. Therapie. 2004;59:483–4. doi: 10.2515/therapie:2004083. [DOI] [PubMed] [Google Scholar]
- 3.Khan MA. Chemical composition and medicinal properties of Nigella sativa Linn. Inflammopharmacology. 1999;7:15–35. doi: 10.1007/s10787-999-0023-y. [DOI] [PubMed] [Google Scholar]
- 4.D’Antuono LF, Moretto A, Antoine FS, Lovato A. Seed yield, yield components, oil cintent and essential oil cotent and composition of nigella saliva and Nigella damascenal. Industrial crops and products. 2002;15:59. [Google Scholar]
- 5.Merfort I, Wray KN, Barakat M, Sam M, Hussein S, M.A.M. Nawwar. Flavonol triglycosides from seeds of Nigella sative. Phytochemistry. 1997;46:359–63. [Google Scholar]
- 6.Tian Z, Liu YM, Chen SB, Yang JS, Xiao PG, Wang L, et al. Cytotoxicity of two triterpenoids from Nigella glandulifera. Molecules. 2006;11:693–9. doi: 10.3390/11090693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Porter KE, Turner NA, O’Regan DJ, Ball SG. Tumor necrosis factor alpha induces human atrial myofibroblast proliferation, invasion and MMP-9 secretion: Inhibition by simvastatin. Cardiovasc Res. 2004;64:507–15. doi: 10.1016/j.cardiores.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 8.Kim S, Choi JH, Kim JB, Nam SJ, Yang JH, Kim JH, et al. Berberine suppresses TNF-alpha-induced MMP-9 and cell invasion through inhibition of AP-1 activity in MDA-MB-231 human breast cancer cells. Molecules. 2008;13:2975–85. doi: 10.3390/molecules13122975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le KH, Graham L, Miller CH, Kmieciak M, Manjili MH, Bear HD. Incubation of antigen-sensitized T lymphocytes activated with bryostatin 1 + ionomycin in IL-7 + IL-15 increases yield of cells capable of inducing regression of melanoma metastases compared to culture in IL-2. Cancer Immunol Immunother. 2009;58:1565–76. doi: 10.1007/s00262-009-0666-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Voutsas IF, Baxevanis CN, Gritzapis AD, Missitzis I, Stathopoulos GP, Archodakis G, et al. Synergy between interleukin-2 and prothymosin alpha for the increased generation of cytotoxic T lymphocytes against autologous human carcinomas. Cancer Immunol Immunother. 2000;49:449–58. doi: 10.1007/s002620000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Man S, Bocci G, Francia G, Green SK, Jothy S, Hanahan D, et al. Antitumor effects in mice of low-dose (metronomic) cyclophosphamide administered continuously through the drinking water. Cancer Res. 2002;62:2731–5. [PubMed] [Google Scholar]
- 12.Tao X, Tang D. Advances in the study of tumor angiogenesis and anti-angiogenic Chinese herbal drugs. Zhong Yao Cai. 2003;26:379–81. [PubMed] [Google Scholar]
- 13.Goel HC, Agrawala PK, Pathania V, Malhotra N. Immunomodulatory and cytoprotective role of RP-1 in gamma-irradiated mice. Mol Cell Biochem. 2003;254:73–81. doi: 10.1023/a:1027308230204. [DOI] [PubMed] [Google Scholar]
- 14.Magalhaes HI, Veras ML, Torres MR, Alves AP, Pessoa OD, Silveira ER, et al. In-vitro and in-vivo antitumour activity of physalins B and D from Physalis angulata. J Pharm Pharmacol. 2006;58:235–41. doi: 10.1211/jpp.58.2.0011. [DOI] [PubMed] [Google Scholar]
- 15.Goyal M, Ganguly NK, Mahajan RC. Immunological response in experimentally reactivated toxoplasmosis in mice. Med Microbiol Immunol. 1989;178:269–78. doi: 10.1007/BF00191061. [DOI] [PubMed] [Google Scholar]


