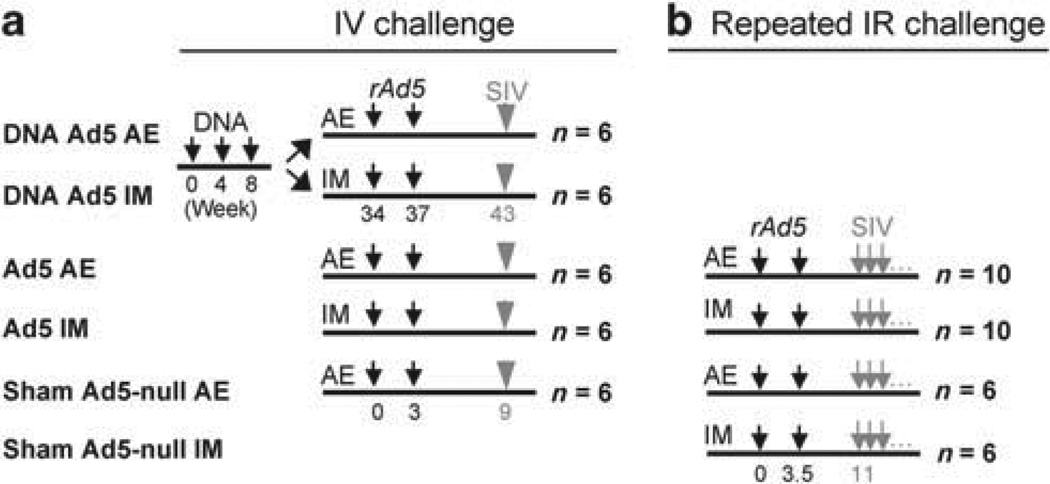
Figure 1.
Experimental schema for the SIVmac251 challenge studies. (a) In the intravenous (IV) study, animals were immunized (small black arrows) with the indicated vaccine component (DNA or rAd5) at the indicated week. SIV challenge (gray arrows) was administered 6 or 7.5 weeks after the second rAd5 immunization for the IV and limiting repeated intrarectal (IR) challenges (b), respectively. Repeated IR challenges were administered as described in the Methods. Vaccination group names are indicated on the left. AE, aerosol; IM, intramuscular.