Abstract
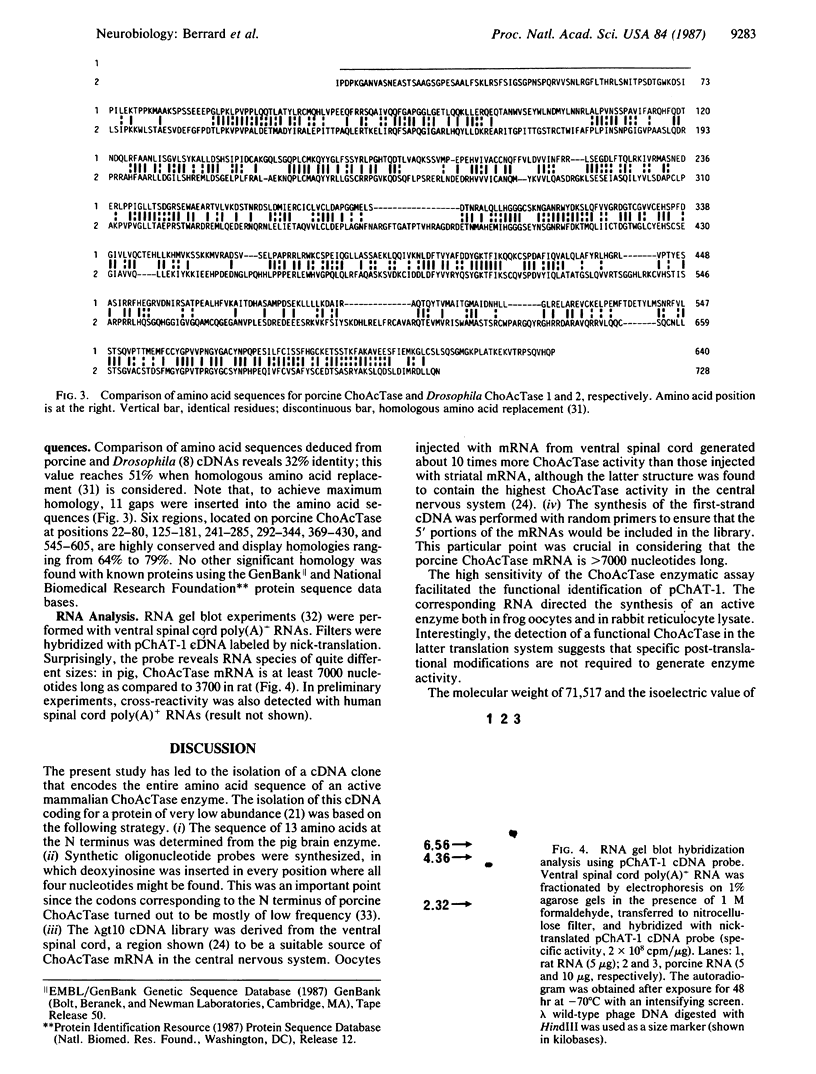
A cDNA clone encoding the complete sequence of porcine choline acetyltransferase (ChoAcTase; acetyl-CoA: choline O-acetyltransferase, EC 2.3.1.6.) has been identified. A cDNA library, constructed from poly(A)+ RNA of ventral spinal cord, was screened with a mixture of eight oligonucleotides corresponding to the N-terminal sequence of pig brain ChoAcTase. Among five positive clones, one, pChAT-1, was identified as a ChoAcTase cDNA clone based on the following criteria. (i) This clone has an open reading frame coding for a protein of the size expected for ChoAcTase (640 amino acids). (ii) The amino acid composition deduced from the nucleotide sequence of this open reading frame matches that of purified porcine ChoAcTase. (iii) When subcloned in the T7 expression system, the corresponding RNA directs the synthesis in the rabbit reticulocyte lysate of a protein that is specifically immunoprecipitated by antibodies raised against ChoAcTase. (iv) Finally and most important, this corresponding RNA, when translated in the reticulocyte lysate, as well as in the Xenopus oocyte system, directs the synthesis of a protein displaying ChoAcTase activity. This activity is inhibited by the specific ChoAcTase inhibitor 4-(1-naphthylvinyl)pyridine. Comparison of porcine ChoAcTase sequence with that of Drosophila reveals 32% identity between these proteins, when the sequences are suitably aligned. pChAT-1 probe hybridizes with a porcine mRNA species that is at least 7000 nucleotides long, whereas the equivalent rat mRNA species is 3700 nucleotides long.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benishin C. G., Carroll P. T. Multiple forms of choline-O-acetyltransferase in mouse and rat brain: solubilization and characterization. J Neurochem. 1983 Oct;41(4):1030–1039. doi: 10.1111/j.1471-4159.1983.tb09047.x. [DOI] [PubMed] [Google Scholar]
- Berrard S., Biguet N. F., Gregoire D., Blanot F., Smith J., Mallet J. Synthesis of catalytically active choline acetyltransferase in Xenopus oocytes injected with messenger RNA from rat central nervous system. Neurosci Lett. 1986 Dec 3;72(1):93–98. doi: 10.1016/0304-3940(86)90625-7. [DOI] [PubMed] [Google Scholar]
- Biguet N. F., Buda M., Lamouroux A., Samolyk D., Mallet J. Time course of the changes of TH mRNA in rat brain and adrenal medulla after a single injection of reserpine. EMBO J. 1986 Feb;5(2):287–291. doi: 10.1002/j.1460-2075.1986.tb04211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black I. B., Patterson P. H. Developmental regulation of neurotransmitter phenotype. Curr Top Dev Biol. 1980;15(Pt 1):27–40. doi: 10.1016/s0070-2153(08)60115-5. [DOI] [PubMed] [Google Scholar]
- Braun A., Barde Y. A., Lottspeich F., Mewes W., Thoenen H. N-terminal sequence of pig brain choline acetyltransferase purified by a rapid procedure. J Neurochem. 1987 Jan;48(1):16–21. doi: 10.1111/j.1471-4159.1987.tb13121.x. [DOI] [PubMed] [Google Scholar]
- Bruce G., Wainer B. H., Hersh L. B. Immunoaffinity purification of human choline acetyltransferase: comparison of the brain and placental enzymes. J Neurochem. 1985 Aug;45(2):611–620. doi: 10.1111/j.1471-4159.1985.tb04030.x. [DOI] [PubMed] [Google Scholar]
- Cohen P. The role of protein phosphorylation in the hormonal control of enzyme activity. Eur J Biochem. 1985 Sep 16;151(3):439–448. doi: 10.1111/j.1432-1033.1985.tb09121.x. [DOI] [PubMed] [Google Scholar]
- Coyle J. T., Price D. L., DeLong M. R. Alzheimer's disease: a disorder of cortical cholinergic innervation. Science. 1983 Mar 11;219(4589):1184–1190. doi: 10.1126/science.6338589. [DOI] [PubMed] [Google Scholar]
- Deininger P. L. Random subcloning of sonicated DNA: application to shotgun DNA sequence analysis. Anal Biochem. 1983 Feb 15;129(1):216–223. doi: 10.1016/0003-2697(83)90072-6. [DOI] [PubMed] [Google Scholar]
- Dobberstein B., Garoff H., Warren G., Robinson P. J. Cell-free synthesis and membrane insertion of mouse H-2Dd histocompatibility antigen and beta 2-microglobulin. Cell. 1979 Aug;17(4):759–769. doi: 10.1016/0092-8674(79)90316-7. [DOI] [PubMed] [Google Scholar]
- Dudley J. P., Butel J. S., Socher S. H., Rosen J. M. Detection of mouse mammary tumor virus RNA in BALB/c tumor cell lines of nonviral etiologies. J Virol. 1978 Dec;28(3):743–752. doi: 10.1128/jvi.28.3.743-752.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckenstein F., Barde Y. A., Thoenen H. Production of specific antibodies to choline acetyltransferase purified from pig brain. Neuroscience. 1981;6(6):993–1000. doi: 10.1016/0306-4522(81)90065-8. [DOI] [PubMed] [Google Scholar]
- Eckenstein F., Thoenen H. Production of specific antisera and monoclonal antibodies to choline acetyltransferase: characterization and use for identification of cholinergic neurons. EMBO J. 1982;1(3):363–368. doi: 10.1002/j.1460-2075.1982.tb01175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eder-Colli L., Amato S. Membrane-bound choline acetyltransferase in Torpedo electric organ: a marker for synaptosomal plasma membranes? Neuroscience. 1985 Jun;15(2):577–589. doi: 10.1016/0306-4522(85)90235-0. [DOI] [PubMed] [Google Scholar]
- Grima B., Lamouroux A., Blanot F., Biguet N. F., Mallet J. Complete coding sequence of rat tyrosine hydroxylase mRNA. Proc Natl Acad Sci U S A. 1985 Jan;82(2):617–621. doi: 10.1073/pnas.82.2.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grima B., Lamouroux A., Boni C., Julien J. F., Javoy-Agid F., Mallet J. A single human gene encoding multiple tyrosine hydroxylases with different predicted functional characteristics. Nature. 1987 Apr 16;326(6114):707–711. doi: 10.1038/326707a0. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hersh L. B., Wainer B. H., Andrews L. P. Multiple isoelectric and molecular weight variants of choline acetyltransferase. Artifact or real? J Biol Chem. 1984 Jan 25;259(2):1253–1258. [PubMed] [Google Scholar]
- Itoh N., Slemmon J. R., Hawke D. H., Williamson R., Morita E., Itakura K., Roberts E., Shively J. E., Crawford G. D., Salvaterra P. M. Cloning of Drosophila choline acetyltransferase cDNA. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4081–4085. doi: 10.1073/pnas.83.11.4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 1984 Sep 25;12(18):7057–7070. doi: 10.1093/nar/12.18.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamouroux A., Faucon Biguet N., Samolyk D., Privat A., Salomon J. C., Pujol J. F., Mallet J. Identification of cDNA clones coding for rat tyrosine hydroxylase antigen. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3881–3885. doi: 10.1073/pnas.79.12.3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathe R. Synthetic oligonucleotide probes deduced from amino acid sequence data. Theoretical and practical considerations. J Mol Biol. 1985 May 5;183(1):1–12. doi: 10.1016/0022-2836(85)90276-1. [DOI] [PubMed] [Google Scholar]
- Le Douarin N. M. The ontogeny of the neural crest in avian embryo chimaeras. Nature. 1980 Aug 14;286(5774):663–669. doi: 10.1038/286663a0. [DOI] [PubMed] [Google Scholar]
- Leff S. E., Rosenfeld M. G., Evans R. M. Complex transcriptional units: diversity in gene expression by alternative RNA processing. Annu Rev Biochem. 1986;55:1091–1117. doi: 10.1146/annurev.bi.55.070186.005303. [DOI] [PubMed] [Google Scholar]
- Lomedico P. T., Saunders G. F. Preparation of pancreatic mRNA: cell-free translation of an insulin-immunoreactive polypeptide. Nucleic Acids Res. 1976 Feb;3(2):381–391. doi: 10.1093/nar/3.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malthe-Sorenssen D. Molecular properties of choline acetyltransferase from different species investigated by isoelectric focusing and ion exchange adsorption. J Neurochem. 1976 Apr;26(4):861–865. doi: 10.1111/j.1471-4159.1976.tb04463.x. [DOI] [PubMed] [Google Scholar]
- Martin F. H., Castro M. M., Aboul-ela F., Tinoco I., Jr Base pairing involving deoxyinosine: implications for probe design. Nucleic Acids Res. 1985 Dec 20;13(24):8927–8938. doi: 10.1093/nar/13.24.8927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R. B., Woodgett J. R., Cohen P., Kemp B. E. Substrate specificity of a multifunctional calmodulin-dependent protein kinase. J Biol Chem. 1985 Nov 25;260(27):14471–14476. [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Rossier J. Choline acetyltransferase: a review with special reference to its cellular and subcellular localization. Int Rev Neurobiol. 1977;20:283–337. doi: 10.1016/s0074-7742(08)60656-x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slemmon J. R., Salvaterra P. M., Crawford G. D., Roberts E. Purification of choline acetyltransferase from Drosophila melanogaster. J Biol Chem. 1982 Apr 10;257(7):3847–3852. [PubMed] [Google Scholar]
- Smith J., Fauquet M., Ziller C., Le Douarin N. M. Acetylcholine synthesis by mesencephalic neural crest cells in the process of migration in vivo. Nature. 1979 Dec 20;282(5741):853–855. doi: 10.1038/282853a0. [DOI] [PubMed] [Google Scholar]
- White H. L., Cavallito C. J. Choline acetyltransferase. Enzyme mechanism and mode of inhibition by a styrylpyridine analogue. Biochim Biophys Acta. 1970 Jun 10;206(3):343–358. doi: 10.1016/0005-2744(70)90151-8. [DOI] [PubMed] [Google Scholar]



