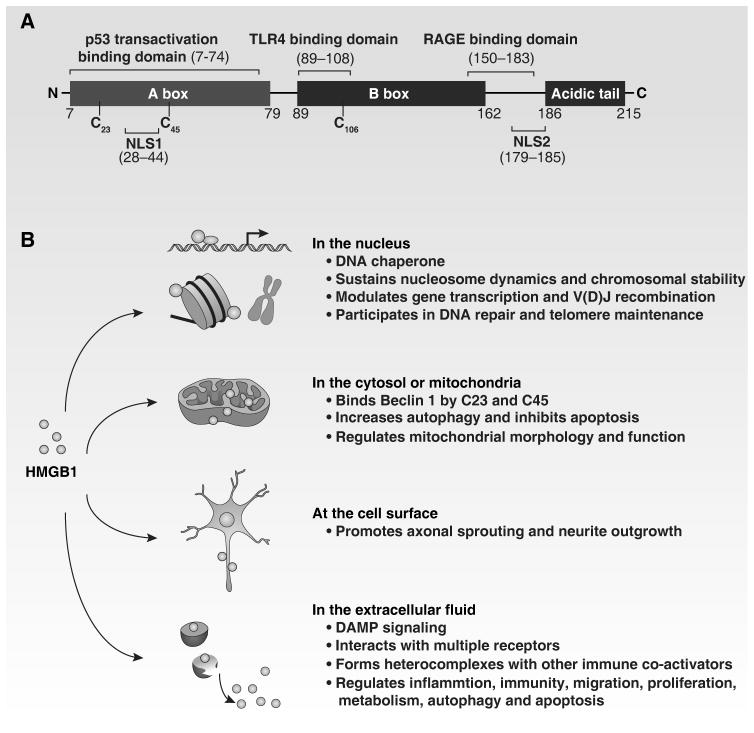
Figure 1. Structure and function of HMGB1.
(A) HMGB1 is structurally composed of three different domains: two homologous DNA-binding domains (box A and box B) and a negatively charged C-terminal domain. Residues 150–183 are responsible for binding to RAGE, whereas residues 89–108 and residues 7–74 are responsible for binding to TLR4 and p53 transactivation domain, respectively. Two nuclear localization signals (NLS1 and NLS2) control nuclear transport of HMGB1. In addition, HMGB1 contains three redox-sensitive cysteine residues (C23, C45 and C106), which are important for HMGB1 activity. (B) HMGB1 has multiple roles based on its location, as indicated.