Abstract
Mononuclear phagocytic cells, including macrophages and dendritic cells, are widely distributed throughout our organs where they perform important homeostatic, surveillance and regenerative tasks. In response to infection or injury, the composition and number of mononuclear phagocytic cells changes remarkably, in part due to the recruitment of inflammatory monocytes from bone marrow. In infection or injury, macrophages and dendritic cells perform important innate and adaptive immune roles from the initial insult through repair and regeneration of the tissue and resolution of inflammation. Evidence from mouse models of disease has shown increasing complexity and subtlety to the mononuclear phagocytic system, which will be reviewed here. New studies show that in addition to monocytes, the resident populations of mononuclear phagocytes expand in disease states and play distinct but important roles in the immune response. Finally, new insights into these functionally diverse cells are now translating into therapeutics to treat human disease.
Introduction
The mononuclear phagocyte system (MPS) generated from hematopoietic stem cells in the bone marrow encompasses monocytes, macrophages and dendritic cells (DC). Recent studies indicate there are overlapping definitions of macrophages and DCs developed from either historically separate research tracks or absence of experimental tools to adequately discriminate these cells. Macrophages and DCs have common and distinct functions. Both cell types are able to phagocytize, migrate, present antigens, release cytokines and chemokines, and mediate anti-inflammatory/immunosuppressive effects [1]. Activated phenotypes can develop into distinct subpopulations with different functions, demonstrating the functional plasticity of macrophages and DCs. Therefore during immune responses, differentiated MPS cells play distinct roles, but ones that all contribute to host defense. Hence, MPS cells function in both innate and adaptive responses to injury or infection, as well as during the resolution phases of tissue inflammation and repair. Why myeloid cells have evolved this dual role in immune response and how this is regulated remain to be fully understood [2, 3].
Current understanding of defining Mononuclear Phagocyte System
The mononuclear phagocyte system (MPS) encompasses monocytes, macrophages and dendritic cells (DC) and has pivotal roles in inflammation, autoimmunity, infection, cancer and organ transplantation, among many other homeostatic and diseases processes. Commitment to the MPS is determined when bone marrow precursors differentiate into the macrophage and DC common progenitors (MDP). At this stage, erythroid, megakaryocyte, lymphoid and granulocyte commitment has been precluded, and MDPs differentiate into common DC progenitors (CDP) or monocytes. CDPs further commit to plasmacytoid DCs or preDCs resulting in DCs (CD8 positive and negative) in lymphoid tissue or CD103+CD11b− DCs in non-lymphoid tissue. Monocytes are able to give rise to macrophages or DCs (CD11b+CD103−) in non-lymphoid tissue. CD8+ and CD11b+ DCs equivalents with a distinct gene expression profile (e.g. BDCA3 and BDCA1, respectively) are also found in lymphoid and non-lymphoid tissues in humans [4].
It is widely accepted that the MPS is generated from hematopoietic stem cells (HSC) in the bone marrow. However, in addition to bone marrow-derived mononuclear phagocytic cells (MPCs), a recent study reported a new lineage of macrophages that develops during the first stages of embryogenesis before the appearance of HSCs. These macrophages express F4/80 at high levels, and traffic early in development to epithelial tissues including liver skin, as well as to brain, to become permanently residing Kupffer cells, Langerhans cells or microglia, respectively, independently of HSCs. Subsets of these cells have been reported in kidney, spleen and lungs. In contrast, marrow-derived MPCs (either from bone marrow or hematopoietic progenitors in embryonic liver and spleen) have been reported to give rise to resident tissue MPCs which express F4/80 at lower levels [5].
Monocytes released from the bone marrow circulate in the blood and migrate into tissues throughout the body. Under steady state conditions, these cells have been thought to differentiate into resident macrophages, which are essential for tissue homeostasis through phagocytosis of apoptotic bodies and production of growth factors (Figure 1). The extent to which monocytes become permanently residing cells of peripheral organs in homeostasis remains somewhat controversial since studies that demonstrated this have used irradiation to replace bone marrow stem cells with tagged cells, and such irradiation may also reduce the number of resident cells, thereby creating a requirement for replacement that is not normally present. Furthermore it is clear that resident MPCs have the capacity for self-renewal [6]. Nevertheless, it is likely that at least a proportion of resident MPCs are repopulated by circulating monocytes. Other studies have suggested that monocytes do not serve as a direct precursor for peripheral tissue DCs in steady state conditions, whereas in conditions of inflammation in vivo or exposed to inflammatory cytokines in vitro monocytes become inflammatory mononuclear phagocytes (MPC) with both phenotypic characteristics of DCs and macrophages [7]. Cheong et al. challenged mice with lipopolysaccharide or live or dead gram-negative bacteria to demonstrate DCs are monocyte-derived following microbial stimulation in vivo [8]. Similarly, in lungs monocytes give rise to alveolar DCs [9]. Further, monocyte derived TNF/iNOS-producing (Tip)-DC subsets are recruited into spleens after microbial infection. These cells have the ability to prime T-cells and express co-stimulatory molecules and MHC class II [10]. Ly6Chi monocytes give rise to these cells dependent on CC-Chemokine receptor 2 (CCR2) [11].
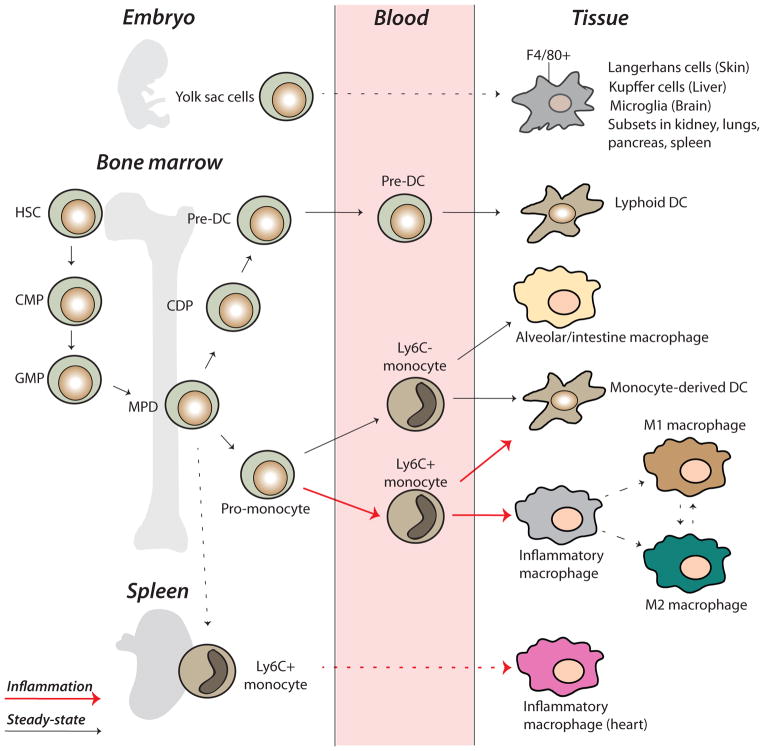
Figure 1.
Origin and development of the mononuclear phagocyte system. The mononuclear phagocyte system (MPS) encompasses monocytes, macrophages and dendritic cells (DC). The MPS is generated from hematopoietic stem cells (HSC) in the bone marrow, but a recent identified new lineage of macrophages (F4/80+) develops from the yolk sac before the appearance of HSCs. These macrophages traffic to epithelial tissues to become permanently residing cells, e.g., Kupffer cells, Langerhans cells or microglia. Subsets are also found in kidney, spleen and lungs. Bone marrow precursors are differentiated into the macrophage and DC common progenitors (MDP) that give rise to common DC progenitors (CDP) or monocytes. CDPs further commit to plasmacytoid DCs or preDCs resulting in DCs. Monocytes are able to give rise to macrophages or DCs. In steady state conditions Ly6Clow monocytes remain around the vascular endothelium. In inflammation Ly6C high monocytes are recruited and differentiate into inflammatory macrophages. The spleen supplies Ly6Chigh monocytes to inflamed heart after myocardial infarction.
Once released from bone marrow, monocytes differentiate in two distinct populations. In steady state conditions, Ly6Clow monocytes reside around the vascular endothelium and patrol within the blood vessel lumen. Tissue macrophages may also derive from Ly6Clow monocytes.
In inflammation, Ly6Chi monocytes are recruited and differentiate into inflammatory macrophages. Although Ly6C is a useful marker in mice, the equivalent marker in humans is CD59 and is not useful as a marker of monocyte subpopulations. It is most likely that in humans these subpopulations of monocytes are defined by CD14+, CD16−, also referred as classical monocytes [3, 12, 13]. Human counterpart for Ly6Clow monocytes are CD14−CD16+ or CD14low CD16+, also called non-classical monocytes [3].
Ly6Chi mouse monocytes express CC-Chemokine receptor 2 (CCR2) and therefore respond to CC-chemokine ligands (CCL) including CCL2, an important chemokine expressed by most cells in response to inflammatory stimuli. By contrast, Ly6Clow monocytes do not express CCR2. In mice the bone marrow serves as a reservoir for Ly6Chi monocytes, and in some studies of heart disease the spleen has been reported to serve as an important additional reservoir for Ly6Chi monocytes, both sites enabling rapid increases in circulating Ly6Chi monocytes in response to injury or infection. After myocardial infarction Ly6Chigh monocyte recruitment from spleen has been proposed to depend on angiotensin II release [14, 15], although it remains unclear whether splenic monocytes traffic to any other organs.
Macrophage subsets
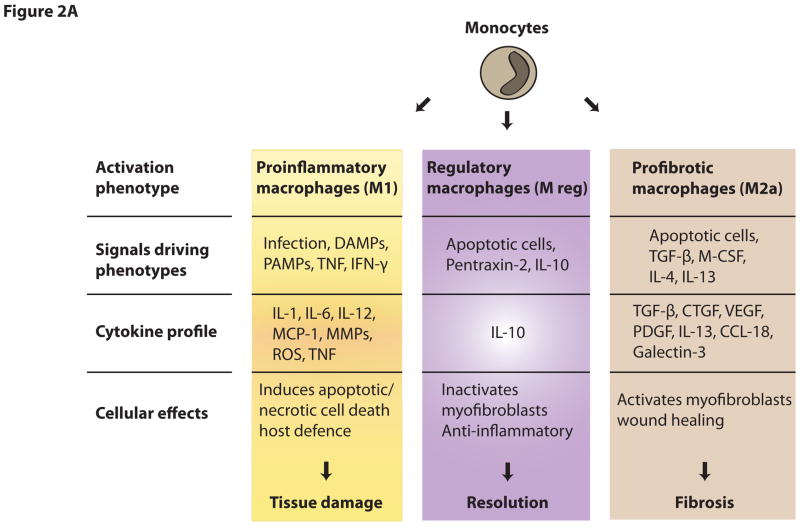
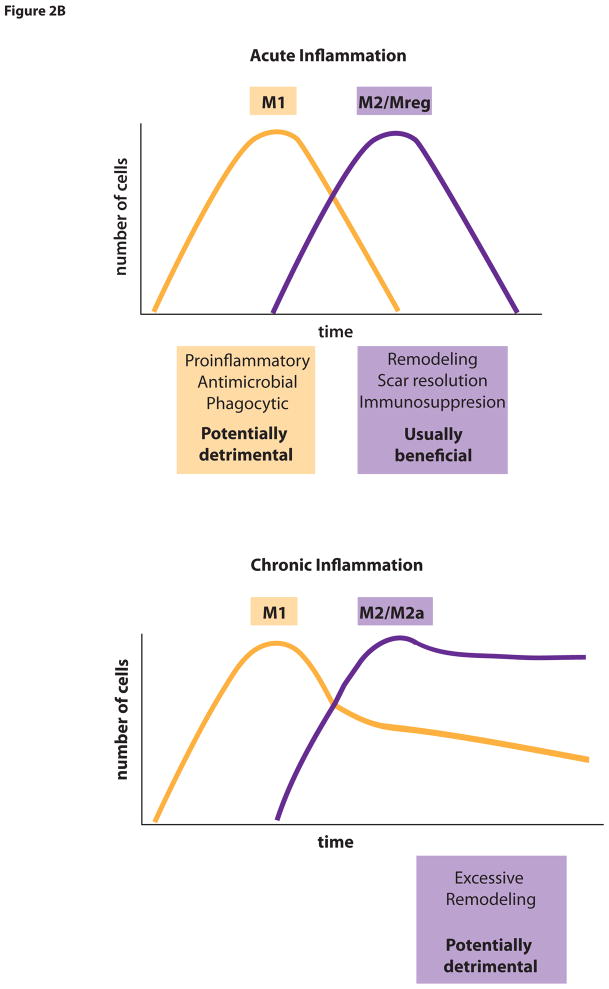
Monocytes, macrophages and DCs, collectively known as mononuclear phagocytic cells (MPCs) are not homogenous populations and can differentiate into distinct functional subsets, which can be shaped by signals encountered within their microenvironment. In response to distinct inflammatory signals, macrophages can differentiate into classically activated (M1) macrophages or other activated macrophages often referred to under an umbrella term alternatively-activated or M2 macrophages. Classically activated M1 macrophages are stimulated by TLR ligands and Th1 immune factors, such as IFN-γ, and mediate host defense from various pathogens including bacteria, viruses or protozoa. They express high levels of proinflammatory cytokines, produce reactive oxygen species, nitric oxide and promote Th1 and Th17 responses [2, 16]. Hence, M1 macrophages are involved in initiating and sustaining inflammatory processes. In contrast, alternatively activated M2 macrophages are considered to have anti-inflammatory functions and promote tissue repair and remodeling. They are also involved in host defense against parasite infection. Furthermore, M2 cells show efficient phagocytic activity, high expression of scavenger receptors, expression of mannose and galactose receptors and have different chemokine expression profiles compared with M1 macrophages. Alternatively activated M2 macrophages comprise a wide spectrum of macrophage functions that differ from acute inflammation (i.e., M1 type functions). These functions include resolution of inflammation, cytoprotection, promotion of angiogenesis, tissue regeneration, matrix remodeling and matrix resorption. In some circumstances promotion of matrix remodeling may become a detrimental process. There is no consensus as to whether these subtypes of macrophages are simply functional states or distinct subpopulations, but evidence exists that one function of macrophages can be switched into another and that macrophage functions can temporally change in disease. Because M2 macrophages exhibit extensive diversity, those macrophages, which perform cytoprotective, immunospressive repair/regeneration functions may be termed ‘regulatory’ macrophages (Mreg) [16]. Mreg express mannose and scavenger receptors at high levels but differ in chemokine and cytokine expression profile from other M2 macrophages, which stimulate matrix deposition, and M1 type macrophages. They are induced by signals including IL-10, glucocorticoid hormones, apoptotic danger associated molecules (DAMPs) and immune complexes (Figure 2A). These functions served by Mreg are usually beneficial for prevention of tissue damage and the re-establishment of tissue homeostasis. In liver injury, M2 macrophages degrade and resorb fibrotic extracellular matrix, using proteinases, such as matrix metalloproteinases (MMP), collagen endocytic receptors and the lysosomal pathway. In contrast, in chronic inflammation, other types of M2 macrophages exist but these stimulate excessive tissue remodeling which results in fibrosis (Figure 2B). Such macrophages (named M2a in some literature) generate factors including PDGFs, TGFβ1 and IGFs that may stimulate mesenchymal cells to deposit fibrous matrix and undergo contraction. Thus, macrophages have one of two major phenotypes. Either they induce host defense and inflammatory response or suppress these functions. Consequently, macrophages can have active roles in both induction and resolution of immune responses, similar to the well-established function of subsets of T cells.
Figure 2.
Figure 2A. Functional phenotypes of macrophage subpopulations. Dependent on the microenvironment macrophages can differentiate into specific subpopulations with distinct phenotype and function.
Figure 2B. Macrophage polarization in acute and chronic inflammation. Mreg macrophages are usually beneficial for prevention of tissue function as immunosuppressant and in resolution in acute inflammation. In chronic inflammation, other types of M2 macrophages (M2a) stimulate excessive tissue remodeling, which results in fibrosis.
Explaining Phenotypic Divergence of mononuclear phagocyte cells
It is unknown whether the M1 and M2 switch occurs at the stage of recruitment of circulating precursor cells or a re-education of already differentiated cells in the tissue. Although there are differences among the M2/Mreg subsets, all of them mainly have immunosuppressive activity. To understand this dichotomous behavior several hypotheses have been proposed: One hypothesis is that macrophages become polarized by cytokine exposure to an M1 (pro-injurious phenotype) or an M2 macrophage (anti-inflammatory). Based on in vitro studies, in which cultured macrophages are simulated by individual cytokines M2 cells have been further divided into M2a (profibrotic) or M2b (angiogenic) or M2 regulatory (Mreg) phenotype (Figure 2A). Although this hypothesis has merit, it derives from in vitro studies and recent insights into behavior of macrophages in vivo suggest that this hypothesis may not hold true in vivo. This hypothesis proposes that the initial cytokine exposure of the monocyte dictates a polarized phenotype and that the major cytokines that stimulate an M2 type phenotype are IL-4 or IL-13. However, in some tissues the expression of these cytokines is extremely low or undetectable during sterile inflammation, yet macrophages with M2-like properties are present. For example, M2a type macrophages readily appear in the chronically inflamed kidney in the absence of IL-4 or IL-13, and they derive largely from M1 type macrophages. Furthermore, in vitro, TLR activated macrophages deactivate proinflammatory cytokine production after 24h and become M2-like, suggesting that the acquisition of anti-inflammatory properties may be intrinsic to macrophages after M1 activation.
A second hypothesis is that there are several populations of distinct myeloid cells in the injured tissues performing different functions. For example, there is a large population of resident dendritic cells and macrophages in the kidney that responds quite distinctly from monocytes to a range of activating stimuli. It is possible that the resident pool of cells serves cytoprotective or reparative functions, whereas macrophages derived from circulating inflammatory monocytes perform mainly M1 type functions.
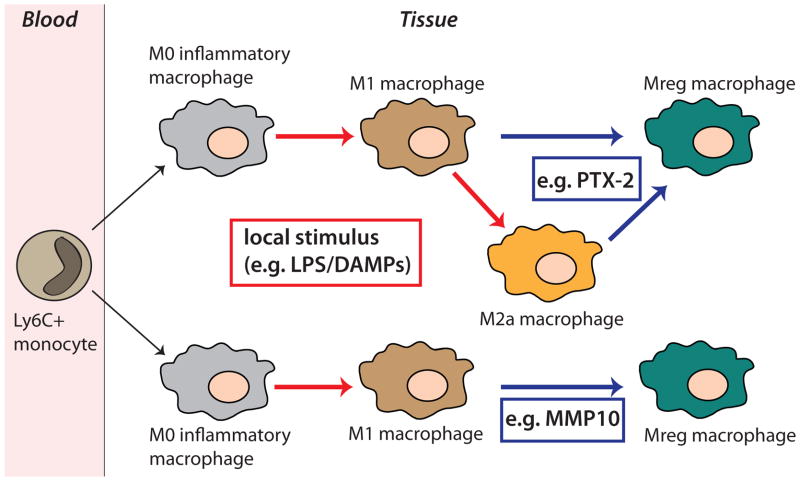
A third hypothesis is that there are discrete functional populations that can switch from one form to the other as regulatory macrophages can differentiate from M1 and M2 (wound-healing) activated macrophages, triggered by mechanisms that are unresolved at present. This macrophage switch results in macrophage phenotype generating high levels of IL-10 and therefore actively suppressing immune responses [17]. There is evidence of macrophage reprograming in vivo. It was shown that co-ligation of certain receptors on the monocyte surface can re-program monocytes during their activation by innate immune stimuli (Figure 3). For example, ligation of Fcγ-receptors by the pentraxin protein 2 (PTX-2) induced a phenotype switch during activation to become a high IL-10/low IL-1β producing monocyte in vivo. Therefore, PTX-2 partially reprograms inflammatory macrophages toward a reparative/anti-inflammatory phenotype in renal fibrosis model (unilateral ureteral obstruction) thereby preventing fibrogenesis [18].
Figure 3.
Macrophage reprogramming in vivo. During activation by innate immune stimuli co-ligation of certain macrophage receptors can induce a phenotype conversion of the different types of inflammatory macrophages. Pentraxin 2 reduces fibrosis and promotes resolution by stimulating phenotype switch towards Mreg macrophages. MMP10 inhibits inflammation and tissue damage by stimulating M1 to Mreg macrophage conversion.
There is accumulating evidence that the WNT pathway could be a target for macrophage phenotype switching. β-catenin is expressed in hematopoietic stem cells, macrophages, DCs and lymphocytes [19] and exposure of human alveolar macrophages or peripheral blood mononuclear cells (PBMCs) to LPS results in accumulation and transcriptional activity of β-catenin [20, 21]. Recently, glycogen synthase kinase 3β (GSK3β), a natural inhibitor of canonical WNT signaling, has been shown to modulate inflammatory responses. After GSK3β inhibition, which activates β-catenin, stimulation of monocytes or PBMCs with various TLR agonists induced IL-10 production while suppressing the release of proinflammatory cytokines [22]. In intestinal DCs, β-catenin is needed for the expression of anti-inflammatory cytokines such as IL-10 and TGFβ1. In addition, mice with conditional ablation of β-catenin in DCs showed increased inflammation in a mouse model of bowel disease [23]. Wnt7b that is produced by macrophages has also critical function for kidney repair and regeneration [24].
A recent study about pro-resolving lipid mediators showed different human macrophage subtypes with distinct lipid mediator profile. Uptake of apoptotic neutrophils by M1 or M2 macrophages induced a different modulation of their lipid mediator profile, respectively. These data indicate a microparticle regulation of specific endogenous lipid mediators during acute inflammation and their possible role in phenotype change in macrophages [25]. The transcription factor IRF5 seems to play a critical role in M1 macrophage polarization. The expression of IRF5 was associated with M1 macrophages and could be induced by inflammatory stimuli [26]. Further, MMP-10 (stromelysin-2) inhibits inflammation and tissue damage in a mouse model of colitis. MMP10 deficiency leads to exacerbated inflammatory responses associated with a phenotype change in macrophages towards M1 and decrease of M2 cells. Therefore, MMP10 could promote repair function by stimulating M1 to M2 conversion in the gut [27]. In the lungs lack of MMP10 exacerbates lung infection 48h after infection with Pseudomonas aeruginosa linked with a significant decrease of IL-10 (unpublished data) (Figure 3).
What is the role of IL-10? IL-10 is a cytokine with anti-inflammatory function and has a central role in the regulation of immune responses. It attenuates inflammation and thereby prevents damage to the host. To inhibit inflammatory disorders, IL-10 functions at different phases of the immune response. Many cells, including T and B cells, as well as cells of the innate immunity comprising dendritic cells and macrophages, express IL-10. IL-10 production can be induced by Toll-like receptor (TLR) or non-TLR ligands in MPC [28]. In many, diverse disease models, IL-10 has been shown to attenuate inflammation. For example, in mouse models of acute kidney injury, IL-10 administration has a beneficial effect by inhibition of leukocyte infiltration and cell death [29]. Therefore, owing to the crucial role of IL-10 in inflammation, a better understanding of the molecular expression of this cytokine and its role in macrophage phenotype will be pivotal.
Mechanisms of activation
Many macrophage studies are based on in vitro studies. Unfortunately, there are often discrepancies compared with findings in vivo. In the steady state tissue macrophages are not active. If tissue macrophages reacted with similar pro-inflammatory responses to those that can be demonstrated in vitro, we might expect to experience a persistent systemic cytokine production. In vivo, therefore, different macrophage activation states may exist, and there are four phases of orderly inflammation: 1. Recruitment to tissue, 2. Differentiation and activation in situ, 3. Switching to suppressive cells and 4. Re-establishment of tissue homeostasis or chronic inflammation [30]. Macrophages play a role in innate and adaptive immunity [16]. They express innate pattern recognition receptors like TLRs, C-type lectin receptors, RIG-like receptors and NOD-like receptors. These receptors recognize microbial stimuli (PAMPs) as well as non-microbial sterile activators (DAMPs) of the innate immune system. Once activated, macrophages release proinflammatory cytokines like TNFα, IL-1β, IL-12, IL-23 and others. Due to their phagocytic ability they can up take and kill foreign material and bacteria.
Macrophages also function as antigen-presenting cells like DCs. Release of proinflammatory cytokines, particularly TNF, IL-12, IL-23 lead to T cell polarization, to antigen specific Th1 cell and Th17 cell responses. A positive feedback loop with T cells releasing IFN-γ drives M1 phenotype and their antimicrobial activity. Later an anti-inflammatory feedback mechanism like IL-10 will leads finally to decision between chronic inflammation and re-establishment of homeostasis.
Resident versus infiltrating macrophages
Macrophages are distributed in all tissues throughout the body and have distinct functions influenced by their location in the body. Resident macrophages in the steady state have numerous roles in tissue homeostasis besides their protective function. Unfortunately there is still no specific marker to distinguish infiltrating from resident macrophages.
Tissue macrophages are basically anti-inflammatory, exhibit high or increased tendency to produce IL-10, whereas infiltrating monocytes are primarily proinflammatory driven by feed forward cytokine networks. Dependent on their stimulus recruited monocytes differentiate into M1 and M2 phenotypes.
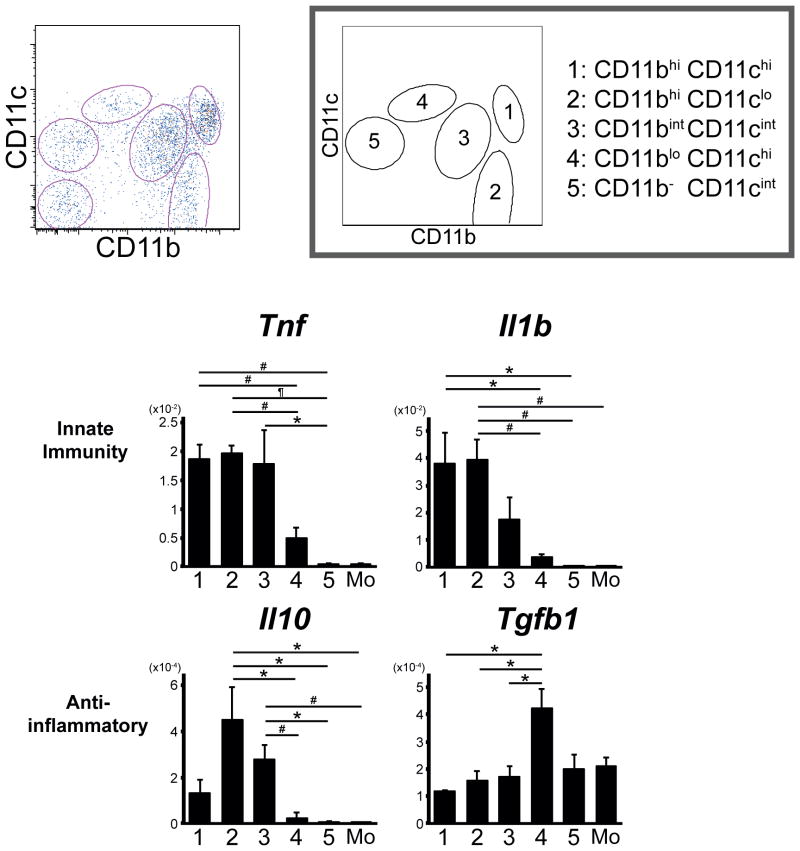
A recent study analyzing resident MPC in the normal kidney revealed five functionally distinct subpopulations (Figure 4) (Kawakami et al., in revision). At steady state they show different surface patterns in surface markers, chemokine expression and gene expression profiles. They also respond to LPS stimulation with a different cytokine profile. Among these subpopulations CD11bintCD11cintF4/80high cells are the most abundant and showed increased capacity to produce IL-10 over monocytes and the other subpopulations (Figure 4). Further is likely that these resident CD11bintCD11cintF4/80high MPCs are derived from yolk sac progenitors and their development dependent on the transcription factor PU.1 [5] and the myeloid receptor colony stimulating factor (CSF) 1 receptor [31]. Although some of these resident subpopulations exit the kidney in response to tissue injury [1], it seems that some remain and expand in inflammatory states [32], and may therefore represent an important population of high IL-10 producing cells that may be responsible for resolution of inflammation and cytoprotection. After comparing each of the discrete MPC subpopulations for antigen presentation functions and typical phagocytic functions, another conclusion of these studies is drawn that conventional dichotomous functions of macrophages and DCs, phagocytosis and antigen presentation, respectively, are dependent on their environment of the tissue cells rather than restricted to distinct cells, “macrophages” or “DCs”.
Figure 4.
Resident kidney mononuclear phagocytes with functionally different phenotypes. Flow cytometry plots showing five subpopulations in the kidney. Quantitative RT-PCR analysis showed different cytokine expression profile after LPS stimulation.
Current tools to study Mononoclear Phagocyte System function
MPCs exist in distinct subpopulations with functionally different recruitment characteristics, distinct receptor and cytokine repertoires. However, to study these cell subpopulations is sometimes problematic as some surface markers are present on more than one population and many show overlapping expression patterns. The fundamental understandings of MPC biology were achieved using knockout mice deficient in different factors essential for the development of MPC. A mutation in the gene CSF1 demonstrated the impact of this hematopoietic growth factor in macrophage development. FLT3 deficient mice demonstrated the importance of this factor in DC differentiation as these mice showed decreased numbers of DC in lymphoid as well as in non-lymphoid tissue. Studies in which myeloid transcription factors (e.g., Stat3, Stat5, IRF 2, IRF5, IRF 4, IRF 8) were mutated in mice have analyzed the role in regulation of MPS cell differentiation [3, 26].
Other studies have used ablation methods to study MPS cells. For example depletion of MPS cells can be achieved by administrating clodronate-encapsulated liposomes that induce apoptosis once they are internalized. This treatment affects multiple MPS cell populations due to their inherent phagocytic activity. Clodronate has minimal toxicity and cell depletion in various tissues is dependent on the administration route. Since phagocytic cells internalize clodronate liposomes unselectively it is difficult to target a distinct MPS cell subset. Therefore choice of administration route may result in selectivity of targeting different MPS populations. For example intravenous injection of clodronate liposomes reaches MPS populations in the spleen, liver and bone marrow, but also affect monocytes [33]. This should be considered when studying macrophages in tissues in inflammatory mouse models as infiltrating macrophages derive from monocytes. MPS subpopulations are differentially susceptible to clodronate.
Cell ablation studies using transgenes to express of the human high-affinity diphtheria toxin receptor (DTR) under control of CD11c or CD11b promoters led to new insights in MPS research. Reporter mice, usually constructed with an expressing vector of a fluorescent gene (e.g., GFP), allow cell tracking without applying antibodies (e.g., CX3CR1-GFP, CCR2-GFP, CD11c-YFP). A limitation of the DTR-models has been the ability to ablate for only short periods of time due to toxicity and / or neurological side effects resulting from ablation of MPS cells in the CNS that has been reported in some long term-dosing studies. Only irradiated chimeras with DTR expression in the bone marrow have proved useful in this regard. Due to the overlapping expression of CD11c and CD11b in DCs and macrophages, ablations studies using CD11b-DTR and CD11c-DTR have in some cases been misleading in attributing biological functions to macrophages only (CD11b) or DCs only (CD11c). Newer DTR mice that target MPS subsets such as CD169-DTR, Langerin-DTR may prove more useful in dissecting the distinct functions of MPS subsets in the future [3, 34, 35].
To enable greater insights into the role of MPS subsets, in collaboration, we have developed a novel IL-10-beta-lactamase reporter mouse which dynamically reports IL-10 producing cells in in vivo mouse models [36]. This mouse labels M2 reparative macrophages. Still, the development animal models in which genes can be mutated in subsets of MPS cells will advance the field and our understanding of the interrelationships of these cells.
Therapeutic potential of Mononuclear Phagocyte System
Over the last decade, modern tools led to new insights in macrophage biology. In rodent studies the therapeutic potential of targeting macrophages has been demonstrated by many researchers. Now recently these insights are being translated into clinical trials. Pfizer has just started a phase 2 multi-center trial to evaluate the efficacy and safety of a macrophage chemokine CCR2/5 receptor antagonist (PF-04634817; NCT01712061, mechanism of action not disclosed by the company) in adults with type 2 diabetes and diabetic nephropathy. The primary outcome will be the urinary albumin to creatinine ratio after 12 weeks of treatment. It was shown in mice that the administration of an antagonist against the macrophage restricted CCR2, ameliorated diabetic nephropathy in type 2 diabetic db/db mice [37]. Currently, there are several clinical studies on the effects of CC-chemokine ligand 2 (CCL2) inhibition in progress. One is a phase II study testing the Spiegelmer® NOX-E36, a CCL2 inhibitor (Noxxon Pharma AG; NCT01547897) in patients with type 2 diabetes and albuminuria. This inhibitor is a complementary RNA oligonucleotide (L-stereoisomer of RNA), which silences CCL2 by targeting CCL2 transcripts. Another phase II study evaluating the effects of carlumab/CNTO888 (Centocor; NCT00786201), a human monoclonal antibody against CCL2 in patients with idiopathic pulmonary fibrosis has been just completed, but results are not published yet. A further phase I study in patients with idiopathic pulmonary fibrosis recently finished testing PRM-151, a recombinant human serum amyloid P/pentraxin 2 (Promedior; NCT01254409). Hopefully, future studies will realize the potential beneficial use of pentraxin 2 and other therapeutic agents inducing a macrophage polarization towards regulatory macrophages. Targeting CSF-1 receptor inhibits macrophage infiltration and has therapeutic effect in mouse models of arthritis, lupus nephritis, multiple sclerosis, which is a promising therapy for these autoimmune diseases. Further, administration of anti CSF-1R antibody reduced early development in atherosclerotic lesions in mice that may be beneficial in preventing atherosclerosis in future [38]. A novel promising strategy stimulating the regenerative capacity of MPC could be nuclear reprograming in the hematopoietic lineage. Lineage reprogramming experiments demonstrated for example a phenotype switch between monocytes and erythroid-megakaryocytes dependent on overexpression of the transcription factors GATA1 or PU.1. This will lead to more new avenues for cell-based therapies and we can expect more specific treatment options in future [39].
Conclusions
Mononuclear phagocytic cells are distributed throughout the body where they perform important homeostatic, surveillance and regenerative tasks. Progress has been made in the understanding of the origin and development of MPS. Sources of mononuclear cells are not longer confined to hematopoietic stem cells in the bone marrow. A second lineage is now known that comprises resident macrophages derived from yolk sac progenitors. Also the spleen serves as reservoir for macrophages which traffic to inflamed ischemic heart tissue. During inflammation or tissue injury MPC have an essential role in innate and adaptive immune responses to host defense, resolution of inflammation and tissue repair and regeneration. MPCs differentiate into distinct functional subsets dependent on their microenvironment. There is good evidence for macrophage reprogramming in vivo as it is shown for co-ligation with pentraxin 2 or MMP10 in inflammatory conditions. Also in steady state conditions we find functionally distinct phenotypes in resident macrophages. Further studies will hopefully provide more insights of the function and origin of these heterogeneous cells and new treatment options for human diseases.
Highlights.
Evidence for macrophage reprogramming in vivo
Macrophage polarization in acute and chronic inflammation
Resident macrophage subpopulations are functionally different
Therapeutic potential of targeting Mononuclear Phagocyte System
Acknowledgments
The Duffield Lab is funded by grants from the National Institutes of Health (DK84077, DK87389, DK93493, DK94768, NCATS 1UH2 TR000504), Nephcure Foundation, and American Heart Association (12040023). JL is supported by the Deutsche Forschungsgemeinschaft (DFG, Li 2074/1-1).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
special interest (•) or outstanding interest (••)
- 1.Nelson PJ, et al. The renal mononuclear phagocytic system. J Am Soc Nephrol. 2012;23(2):194–203. doi: 10.1681/ASN.2011070680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2(••).Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122(3):787–95. doi: 10.1172/JCI59643. A comprehensive review about macrophage populations and their role in health and disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chow A, Brown BD, Merad M. Studying the mononuclear phagocyte system in the molecular age. Nat Rev Immunol. 2011;11(11):788–98. doi: 10.1038/nri3087. [DOI] [PubMed] [Google Scholar]
- 4.Satpathy AT, et al. Zbtb46 expression distinguishes classical dendritic cells and their committed progenitors from other immune lineages. J Exp Med. 2012;209(6):1135–52. doi: 10.1084/jem.20120030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5(••).Schulz C, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336(6077):86–90. doi: 10.1126/science.1219179. Evidence for a macrophage lineage derived from the yolksac independent from hematopoietic progenitors. [DOI] [PubMed] [Google Scholar]
- 6.Hoeffel G, et al. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J Exp Med. 2012;209(6):1167–81. doi: 10.1084/jem.20120340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu G, et al. Phenotypic and functional switch of macrophages induced by regulatory CD4+CD25+ T cells in mice. Immunol Cell Biol. 2011;89(1):130–42. doi: 10.1038/icb.2010.70. [DOI] [PubMed] [Google Scholar]
- 8.Cheong C, et al. Microbial stimulation fully differentiates monocytes to DC-SIGN/CD209(+) dendritic cells for immune T cell areas. Cell. 2010;143(3):416–29. doi: 10.1016/j.cell.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jakubzick C, et al. Blood monocyte subsets differentially give rise to CD103+ and CD103- pulmonary dendritic cell populations. J Immunol. 2008;180(5):3019–27. doi: 10.4049/jimmunol.180.5.3019. [DOI] [PubMed] [Google Scholar]
- 10.Serbina NV, et al. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19(1):59–70. doi: 10.1016/s1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- 11.Serbina NV, et al. Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol. 2008;26:421–52. doi: 10.1146/annurev.immunol.26.021607.090326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Auffray C, et al. CX3CR1+ CD115+ CD135+ common macrophage/DC precursors and the role of CX3CR1 in their response to inflammation. J Exp Med. 2009;206(3):595–606. doi: 10.1084/jem.20081385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fogg DK, et al. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 2006;311(5757):83–7. doi: 10.1126/science.1117729. [DOI] [PubMed] [Google Scholar]
- 14.Geissmann F, et al. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327(5966):656–61. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swirski FK, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325(5940):612–6. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galli SJ, Borregaard N, Wynn TA. Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nat Immunol. 2011;12(11):1035–44. doi: 10.1038/ni.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duffield JS. Macrophages and immunologic inflammation of the kidney. Semin Nephrol. 2010;30(3):234–54. doi: 10.1016/j.semnephrol.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castano AP, et al. Serum amyloid P inhibits fibrosis through Fc gamma R-dependent monocyte-macrophage regulation in vivo. Sci Transl Med. 2009;1(5):5ra13. doi: 10.1126/scitranslmed.3000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Staal FJ, Luis TC, Tiemessen MM. WNT signalling in the immune system: WNT is spreading its wings. Nat Rev Immunol. 2008;8(8):581–93. doi: 10.1038/nri2360. [DOI] [PubMed] [Google Scholar]
- 20.Monick MM, et al. Ceramide regulates lipopolysaccharide-induced phosphatidylinositol 3-kinase and Akt activity in human alveolar macrophages. J Immunol. 2001;167(10):5977–85. doi: 10.4049/jimmunol.167.10.5977. [DOI] [PubMed] [Google Scholar]
- 21.Thiele A, et al. Regulation and possible function of beta-catenin in human monocytes. J Immunol. 2001;167(12):6786–93. doi: 10.4049/jimmunol.167.12.6786. [DOI] [PubMed] [Google Scholar]
- 22.Martin M, et al. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol. 2005;6(8):777–84. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manicassamy S, et al. Activation of beta-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science. 2010;329(5993):849–53. doi: 10.1126/science.1188510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin SL, et al. Macrophage Wnt7b is critical for kidney repair and regeneration. Proc Natl Acad Sci U S A. 2010;107(9):4194–9. doi: 10.1073/pnas.0912228107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25(••).Dalli J, Serhan CN. Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood. 2012;120(15):e60–72. doi: 10.1182/blood-2012-04-423525. In vivo evidence for macrophage conversion by administration of resolvins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26(•).Krausgruber T, et al. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat Immunol. 2011;12(3):231–8. doi: 10.1038/ni.1990. The authors describe an essential role for IRF5 in M1 macrophage polarization and demonstrate a new function for IRF5. [DOI] [PubMed] [Google Scholar]
- 27(••).Koller FL, et al. Lack of MMP10 exacerbates experimental colitis and promotes development of inflammation-associated colonic dysplasia. Lab Invest. 2012;92(12):1749–59. doi: 10.1038/labinvest.2012.141. In vivo evidence for macrophage reprogramming by administration of MMP10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10(3):170–81. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 29.Deng J, et al. Interleukin-10 inhibits ischemic and cisplatin-induced acute renal injury. Kidney Int. 2001;60(6):2118–28. doi: 10.1046/j.1523-1755.2001.00043.x. [DOI] [PubMed] [Google Scholar]
- 30.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11(11):723–37. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gomez Perdiguero E, Schulz C, Geissmann F. Development and homeostasis of “resident” myeloid cells: The case of the microglia. Glia. 2013;61(1):112–20. doi: 10.1002/glia.22393. [DOI] [PubMed] [Google Scholar]
- 32.Lin SL, et al. Bone marrow Ly6Chigh monocytes are selectively recruited to injured kidney and differentiate into functionally distinct populations. J Immunol. 2009;183(10):6733–43. doi: 10.4049/jimmunol.0901473. [DOI] [PubMed] [Google Scholar]
- 33.van Rooijen N. Liposomes for targeting of antigens and drugs: immunoadjuvant activity and liposome-mediated depletion of macrophages. J Drug Target. 2008;16(7):529–34. doi: 10.1080/10611860802228426. [DOI] [PubMed] [Google Scholar]
- 34.Bennett CL, Clausen BE. DC ablation in mice: promises, pitfalls, and challenges. Trends Immunol. 2007;28(12):525–31. doi: 10.1016/j.it.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 35.Bar-On L, Jung S. Defining dendritic cells by conditional and constitutive cell ablation. Immunol Rev. 2010;234(1):76–89. doi: 10.1111/j.0105-2896.2009.00875.x. [DOI] [PubMed] [Google Scholar]
- 36(•).Bouabe H, et al. Novel highly sensitive IL-10-beta-lactamase reporter mouse reveals cells of the innate immune system as a substantial source of IL-10 in vivo. J Immunol. 2011;187(6):3165–76. doi: 10.4049/jimmunol.1101477. The authors describe a new IL10 reporter mouse that allows tracking of IL-10 producing cells in myeloid and lymphoid cells. [DOI] [PubMed] [Google Scholar]
- 37.Sayyed SG, et al. An orally active chemokine receptor CCR2 antagonist prevents glomerulosclerosis and renal failure in type 2 diabetes. Kidney Int. 2011;80(1):68–78. doi: 10.1038/ki.2011.102. [DOI] [PubMed] [Google Scholar]
- 38.Mouchemore KA, Pixley FJ. CSF-1 signaling in macrophages: pleiotrophy through phosphotyrosine-based signaling pathways. Crit Rev Clin Lab Sci. 2012;49(2):49–61. doi: 10.3109/10408363.2012.666845. [DOI] [PubMed] [Google Scholar]
- 39(•).Hendry CE, Little MH. Reprogramming the kidney: a novel approach for regeneration. Kidney Int. 2012;82(2):138–46. doi: 10.1038/ki.2012.68. Detailed review about new approaches in cell-based thereapies. [DOI] [PubMed] [Google Scholar]