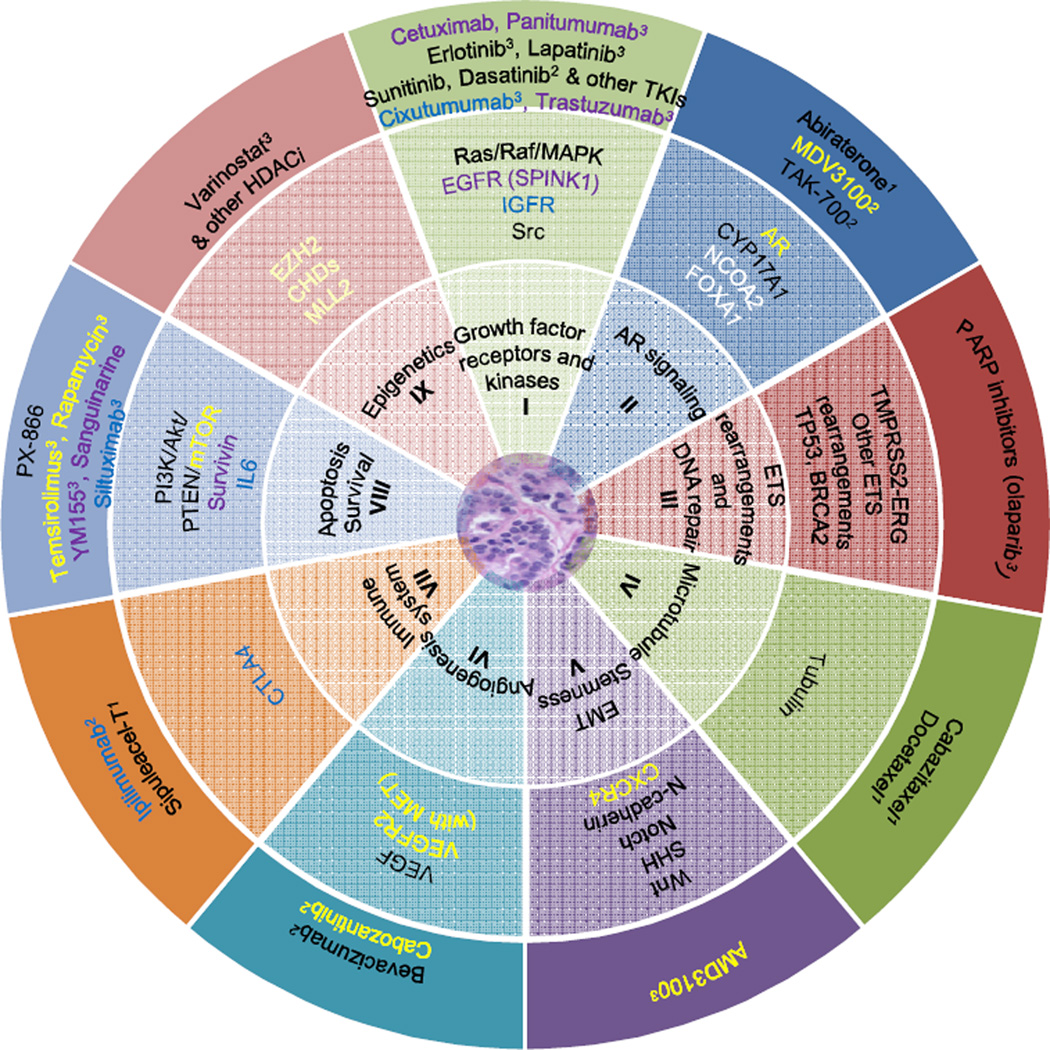
Figure 2. Considerations for targeted therapy based on key pathways perturbed in prostate cancer.
Current standard-of-care involves active surveillance for low-risk localized prostate cancers; hormonal therapy, radical prostatectomy, or radiation therapy for higher-risk localized disease; and androgen pathway suppression for metastatic disease with chemotherapy and immunotherapy at the time of disease progression. This figure shows the potential for targeted therapy in molecularly-defined subtypes of prostate cancer. Genomic alterations are classified based on the class of molecular pathways affected (inner circle). Therapeutic agents (outer circle) targeting respective pathways are grouped with the genes (middle circle) commonly altered in these pathways, coordinated by color wherever possible. Selected agents in various phases of clinical trials are superscripted: 1FDA approved, 2Phase III clinical trials, 3Phase I/II clinical trials; preclinical development not marked. While anti-androgen therapy abiraterone, microtubule inhibitor cabazitaxel, and immunotherapy sipuleacel-T are already in clinical use, aberrations of NCOA2 and FOXA1 genes (white) are recent findings, the functional significance and therapeutic implications of which await further investigation.