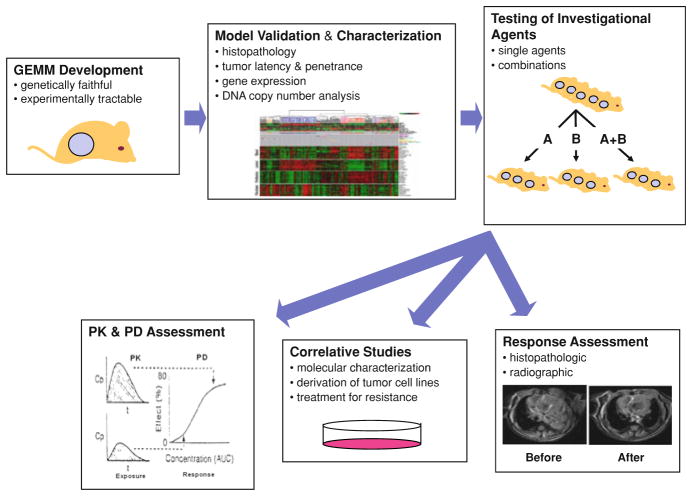
Fig. 2.
Workflow and infrastructural needs for GEMM testing. GEM models are first designed and developed based on genetic events faithful to human cancers. The model is then characterized and validated histopathologically and molecularly prior to therapeutic testing. Once a model is characterized, investigational agents are tested as single agents or in combination. Treated and untreated mice are assessed for tumor response (typically radiographically or other imaging modalities using RECIST-like criteria) with pharmacokinetic (PK) and pharmacodymanic (PD) monitoring. Other correlative studies such as the molecular characterization of treated tumors and culture and in vitro evaluation of GEMM tumor cell lines can be performed as indicated