Abstract
AIM: To evaluate the prognostic factors and efficacy of hepatic arterial infusion chemotherapy in hepatocellular carcinoma with portal vein tumor thrombosis.
METHODS: Fifty hepatocellular carcinoma (HCC) patients with portal vein tumor thrombosis (PVTT) were treated using hepatic arterial infusion chemotherapy (HAIC) via a subcutaneously implanted port. The epirubicin-cisplatin-5-fluorouracil (ECF) chemotherapeutic regimen consisted of 35 mg/m2 epirubicin on day 1, 60 mg/m2 cisplatin for 2 h on day 2, and 500 mg/m2 5-fluorouracil for 5 h on days 1-3. The treatments were repeated every 3 or 4 wk.
RESULTS: Three (6%) of the 50 patients achieved a complete response (CR), 13 (26%) showed partial responses (PR), and 22 (44%) had stable disease (SD). The median survival and time to progression were 7 and 2 mo, respectively. After 2 cycles of HAIC, CR was achieved in 1 patient (2%), PR in 10 patients (20%) and SD in 26 patients (52%). Significant pre-treatment prognostic factors were a tumor volume of < 400 cm3 (P = 0.01) and normal levels of protein induced by vitamin K absence or antagonist (PIVKA)-II (P = 0.022). After 2 cycles of treatment, disease control (CR + PR + SD) (P = 0.001), PVTT response (P = 0.003) and α-fetoprotein reduction of over 50% (P = 0.02) were independent factors for survival. Objective response (CR + PR), disease control, PVTT response, and combination therapy during the HAIC were also significant prognostic factors. Adverse events were tolerable and successfully managed.
CONCLUSION: HAIC may be an effective treatment modality for advanced HCC with PVTT in patients with tumors < 400 cm3 and good prognostic factors.
Keywords: Hepatocellular carcinoma, Hepatic arterial infusion chemotherapy, Portal vein tumor thrombosis
Core tip: The aim of this study was to investigate the prognostic factors of hepatic arterial infusion chemotherapy in advanced hepatocellular carcinoma patients with portal vein tumor thrombosis. The primary findings of this study were as follows: (1) The median survival and time to progression were 7 and 2 mo, respectively; (2) A tumor volume of < 400 cm3 and protein induced by vitamin K absence or antagonist-II were independent pre-treatment prognostic factors; (3) Disease control and ≥ 50% tumor marker reduction were significant prognostic factors after the second cycle of hepatic arterial infusion chemotherapy (HAIC); and (4) Objective tumor response, disease control and portal vein tumor thrombosis response were independent post-treatment prognostic factors at the end of the HAIC.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the sixth most common cancer globally and the third most common cause of cancer mortality[1]. Surveillance of high-risk patients facilitates the early diagnosis of HCC[2]. However, because many patients are diagnosed at intermediate or advanced stages, only 30% of patients benefit from curative therapies such as resection, transplantation, or percutaneous ablation[3]. For patients with vascular invasion and/or extrahepatic spread, i.e., whose tumors were classified as advanced stage according to the Barcelona Clinic Liver Cancer (BCLC) staging system, the multi-kinase inhibitor sorafenib is recommended[4]. In recent randomized controlled trials, sorafenib significantly increased patient survival[5,6]. However, contrary to our expectations, the survival and therapeutic advantages of sorafenib are modest, and the current cost of the drug precludes sorafenib from becoming a more generalized treatment tool for advanced HCC[7]. Systemic chemotherapy also has limited utility in treating HCC due to frequent toxicity and is not associated with improved survival[8,9]. Therefore, alternatives to sorafenib and systemic chemotherapy are often required for the treatment of advanced HCC, and hepatic arterial infusion chemotherapy (HAIC) could be an alternative modality.
HAIC using an implantable port system is theoretically more effective against HCC than systemic chemotherapy. HAIC enables anti-cancer agents to be delivered locally at high concentrations to hypervascular tumors, thereby keeping systemic concentrations of chemotherapeutic agents low due to the first-pass effect[10]. Many studies using HAIC have reported that it is a useful modality for patients with advanced HCC[10-14]. However, there are limited data defining the clinical factors predicting its efficacy. In this study, we investigated the efficacy and predictive factors of HAIC in patients with advanced HCC with portal vein tumor thrombus (PVTT) using the HAIC regimen, which was composed of epirubicin, cisplatin, and 5-fluorouracil.
MATERIALS AND METHODS
Patients
Between March 2009 and January 2012, 68 consecutive patients with advanced HCC underwent HAIC via an implantable port system with epirubicin, cisplatin and 5-fluorouracil (5-FU) in Seoul St. Mary Hospital, Seoul, South Korea. The patients were refractory to previous treatments or not amenable to surgery or locoregional therapies such as ethanol injection, radiofrequency ablation, or transcatheter arterial chemoembolization due to metastasis or PVTT. HCC was diagnosed either histologically or using typical radiologic findings of HCC on two dynamic imaging examinations or one dynamic technique with an elevated serum α-fetoprotein (AFP) level ≥ 200 ng/mL[15,16]. Among these 68 patients, 50 patients who had PVTT and received more than two cycles of HAIC were enrolled in this study. All tumor thromboses were radiologically confirmed in the main trunk or in the first or second branch of the portal vein. Additional inclusion criteria were a white blood cell count ≥ 3000 cells/mm3 or an absolute neutrophil count ≥ 1000 cells/mm3 and a platelet count ≥ 50000 cells/mm3. Other eligibility criteria included the following: ages 18-75 years, Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 1, and a Child-Pugh score ≤ 7. Patients with extrahepatic metastasis were also included in this study because extrahepatic metastasis is common in HCC patients with a large tumor and PVTT due to high AFP levels and large tumor volumes. Exclusion criteria included another concurrent malignancy or other underlying serious medical condition such as renal or cardiopulmonary insufficiency. HCC was staged using the BCLC, modified Union for International Cancer Control (UICC) and American Joint Committee on Cancer (AJCC) staging systems. Tumor volume was measured by a single radiologist using commercially available imaging software (Pinnacle3 with AcQSim3 v. 8.0, Philips, Fitchburg, WI, United States) and the summation-of-areas technique with a 5-mm reconstruction thickness.
Implantation of the arterial port system
After skin preparation and local anesthetic injection, the right common femoral artery was punctured using the Seldinger technique. The superior mesenteric and celiac arteries were selected under fluoroscopic guidance. After the selective angiographies were performed, the right gastric and gastroduodenal arteries were embolized with multiple microcoils to prevent reflux of the cytotoxic drug to the stomach and duodenum. After performing a follow-up celiac arteriography, a catheter was inserted and localized to the proper hepatic artery. The skin and right inguinal region was incised, and the subcutaneous pocket was prepared via dissection. The peripheral end of the catheter was connected to the infusion port, and the port device was implanted in a subcutaneous pocket in the right or left iliac fossa. To prevent the occlusion of the catheter, 10 mL of saline mixed with 10000 units of heparin were locked into the port after each cycle of chemotherapy. Hepatic angiography via the port system was performed every two cycles of treatment.
Chemotherapeutic regimen and additional therapy
The Epirubicin-Cisplatin-5-fluorouracil (ECF) chemotherapeutic regimen included 35 mg/m2 epirubicin on day 1, 60 mg/m2 cisplatin for 2 h on day 2, and 500 mg/m2 5-FU for 5 h on days 1-3. Intravenous hydration was performed prior to cisplatin infusion to prevent nephrotoxicity, and all patients were given prophylactic antiemetic treatment comprised of 5-hydroxytryptamine-3 antagonists. The treatment cycles were repeated every 3 or 4 wk until disease progression, unacceptable toxicity, or patient refusal to continue. The doses of chemotherapeutic agents or treatment intervals were adjusted at every treatment cycle depending on hepatic dysfunction or significant toxicity. The dose of subsequent treatment was reduced by 25% when repeated grade 2 or grade 3/4 toxicity occurred during the preceding cycle[14].
During or after the HAIC treatment, additional therapies were performed as necessary, depending on the tumor responses to HAIC, performance status, and hepatic function. Additional treatment included targeted therapy with sorafenib, external radiation therapy, transarterial chemolipiodolization (TACL), systemic chemotherapy, local therapies such as radiofrequency ablation (RFA) or percutaneous ethanol injection (PEI), or surgical treatment.
Study assessment
The primary endpoints were an objective response rate [complete response (CR) + partial response (PR)] and disease control rate [objective response rate + stable disease (SD)]. Response evaluations were performed after two cycles and at the end of the HAIC treatment. The overall survival (OS) and time to progression (TTP) were evaluated secondarily. The treatment response was classified according to the modified Response Evaluation Criteria In Solid Tumors (mRECIST). The pretreatment evaluation included medical history, physical examination, laboratory tests [complete blood count, blood chemistry, virologic marker, serum AFP and proteins induced by vitamin K absence or antagonist (PIVKA)-II], and imaging studies such as a computed tomography (CT) scan, magnetic resonance imaging (MRI) or positron emission tomography (PET) scan. During the treatment, toxicity assessment, laboratory tests, as well as chest and abdominal X-rays were repeated prior to each treatment cycle. CT scans were performed every two cycles or as needed to evaluate the tumor response or to confirm the disease progression. OS was defined as the time from the first treatment to death or the last follow-up visit, and TTP was the time from the first treatment to the radiologic progression. The patient’s liver function was classified according to the scheme of Child-Pugh. AFP and PIVKA-II reduction were calculated according to the formula [(baseline level-level after the second cycle)/(baseline level) × 100] in patients whose AFP was elevated above 20 ng/mL[17] and PIVKA-II was elevated above 40 mAU/mL[18]. The treatment toxicity was assessed using the Common Terminology Criteria for Adverse Events (CTCAE) v. 4.0[19]. The PVTT response was evaluated using dynamic imaging. Response was defined as complete disappearance or at least a 30% decrease in the diameter of PVTT, and non-response was defined as any case that did not qualify for response.
Statistical analysis
The Kaplan-Meier methods and log-rank tests were used in the analysis of time-to-event variables, and a 95%CI for the median time to event were computed. Cox-proportional hazard regression models were used to determine the hazard ratios (HRs) of pre-treatment and post-treatment prognostic factors for survival. The variables with P values < 0.05 at univariate analysis were used as input variables in the multivariate model using the enter methods. In the multivariate analysis of post-treatment prognostic factors, hazard ratios were adjusted for the significant variables in the multivariate analysis of pre-treatment variables. For each covariate, the proportional hazard assumption was verified using a log minus log survival plot, and Cox-Snell residuals were used to evaluate the fit of the model. A plot of the estimated cumulative hazard rate versus Cox-Snell residuals followed a 45º line.
The χ2-test or Fisher’s exact test were used for the analysis of clinical characteristics and prognostic factors between the disease control group (CR + PR + SD) and the disease progression group [progressive disease (PD)]. Statistical significance was defined as a P value < 0.05. All data were analyzed using the SPSS v. 14.0 software (SPSS, Chicago, IL, United States).
RESULTS
Patients characteristics
The characteristics of the 50 study patients are shown in Table 1. The median age was 54 years (range, 37-74), and 78% of the patients were male. The most common etiology of underlying liver disease was chronic hepatitis B (78%), and 84% had a Child-Pugh classification of A. All patients were BCLC stage C due to PVTT and extrahepatic metastasis, and 30 patients (60%) had main PVTT (Vp4). Sixteen patients (32%) had extrahepatic metastasis at the initiation of HAIC. Twenty-four patients (48%) received previous treatment, and the most common previous treatment was TACL.
Table 1.
Baseline patient characteristics
| Patient characteristics | Statistic |
| Age (yr) | 54 (37-74) |
| Gender (male/female) | 39/11 |
| Etiology | |
| HBV/HCV/non-viral | 39/6/5 |
| Child-Pugh classification | |
| A5/A6/B7 | 17/25/8 |
| Staging | |
| BCLC staging C | 50 |
| Modified UICC (III/IVa/IVb) | 9/31/10 |
| Tumor type | |
| Nodular/massive/infiltrative | 3/4/43 |
| Portal vein thrombosis | |
| Vp2/Vp3/Vp4 | 7/13/30 |
| Maximal tumor size (cm) | |
| < 10/≥ 10 | 26/24 |
| Tumor volume (cm3)1 | 492.7 (26.1-2746.6) |
| Extrahepatic metastases | 16 |
| Previous treatment | |
| TACL/TACL + RFA/TACL + ERT | 19/3/2 |
| Total bilirubin (mg/dL)1 | 0.87 (0.34-1.99) |
| PT (INR)1 | 1.14 (0.94-1.38) |
| ALT (IU/L)1 | 37 (15-345) |
| Platelet count (× 103/mL)1 | 133 (50-326) |
| AFP (ng/mL)1 | 3084.15 (7.94-426100) |
| PIVKA-II (mAU/mL)1 | 1190 (16-12000) |
Expressed as the median (range). AFP: Alpha-fetoprotein; AJCC: American Joint Committee on Cancer; ALT: Alanine aminotransferase; BCLC: Barcelona Clinic Liver Cancer; ERT: External radiation therapy; HBV: Hepatitis B virus; HCV: Hepatitis C virus; PIVKA: Protein induced by vitamin K absence or antagonist; PT: Prothrombin time; RFA: Radiofrequency ablation; TACL: Transarterial chemolipiodolization; UICC: Union for International Cancer Control.
Treatment efficacy
The patients received a total of 289 cycles of HAIC with a median of five cycles (range 2-25 cycles). The response rates are shown in Table 2. After two cycles of HAIC, 11 patients (22%) showed an objective response, and 37 patients (74%) achieved successful disease control. Based on the best response during HAIC, the objective response rate was 32% and disease control rate was 76%.
Table 2.
Tumor responses to hepatic arterial infusion chemotherapy treatment
| Response after two cycles | Best response during HAIC | Overall response after HAIC | Intra-hepatic tumor response | |
| CR | 2% | 6% | 6% | 6% |
| PR | 20% | 26% | 10% | 26% |
| SD | 52% | 44% | 12% | 52% |
| PD | 26% | 24% | 72% | 16% |
| Objective response | 22% | 32% | 16% | 32% |
| Disease control rate | 74% | 76% | 28% | 84% |
HAIC: Hepatic arterial infusion chemotherapy; CR: Complete response; PD: Progressive disease; PR: Partial response; SD: Stable disease.
In total, the median OS was 7 mo (95%CI: 5.5-8.5) and TTP was 2 mo (95%CI: 1.3-2.7), as shown in Figure 1. We assessed the OS according to the presence of objective response and disease control (Figure 2). The treatment responses were evaluated after the second cycle of HAIC and at the end of the HAIC treatment. Based on the best response during HAIC, the median OS was 24 mo (95%CI: 12.9-35.1) in the objective responder group, 5 mo (95%CI: 3.6-6.4) in the non-responder group (P < 0.001), 8 mo (95%CI: 2.6-13.4) in the disease control group and 4 mo (95%CI: 2.9-5.1) in the progressive disease group (P < 0.001). Based on response after the second cycle of HAIC, the median OS was 17 mo (95%CI: 14.6-19.4) in the responder group and 7 mo (95%CI: 5.0-9.0) in the non-responder group (P = 0.034), 11 mo (95%CI: 6.7-15.3) in the disease control group and 5 mo (95%CI: 2.9-5.1) in the progressive disease group (P < 0.001).
Figure 1.
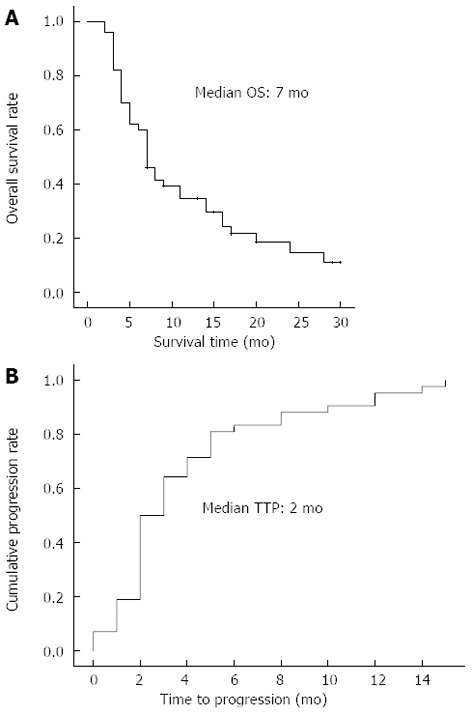
Overall survival rate (A) and time to disease progression (B) of the patients. OS: Overall survival; TTP: Time to disease progression.
Figure 2.
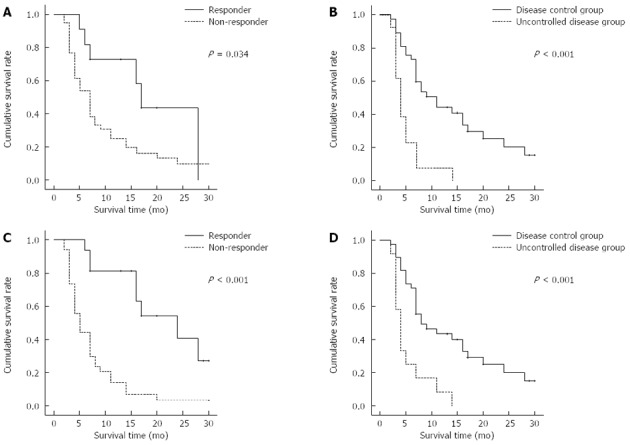
Overall survival of the objective response and disease control groups. A, B: After the second cycle of high-dose hepatic arterial infusion chemotherapy (HAIC); C, D: During HAIC.
Prognostic factors of survival
The prognostic factors affecting patient survival were analyzed by examining the pre-treatment and post-treatment parameters shown in Tables 3 and 4. Univariate analysis revealed that four pre-treatment prognostic factors were significantly associated with survival: tumor volume (< 400 cm3), Child-Pugh score, pre-treatment PIVKA-II level, and AJCC stage. Based on multivariate analysis, a tumor volume of < 400 cm3 [P = 0.01, HR = 2.520 (95%CI: 1.252-5.072)] and PIVKA-II [P = 0.022, HR = 3.121 (95%CI: 1.177-8.274)] were independent prognostic factors among the pre-treatment parameters. There was no significant difference in overall survival according to the presence of extrahepatic metastasis; the median OS was 8 mo (95%CI: 5.192-10.808) in the patients without extrahepatic metastasis and 5 mo (95%CI: 2.387-7.613) in the patients with extrahepatic metastasis (P = 0.201) (Figure 3).
Table 3.
Pre-treatment prognostic factors for survival in hepatic arterial infusion chemotherapy treatment
| Variables | Univariate |
Multivariate1 |
|
| HR (95%CI) | HR (95%CI) | P-value | |
| Age (< 60/≥ 60 yr) | 0.991 (0.514-1.912) | ||
| Gender (male/female) | 1.453 (0.687-3.076) | ||
| Maximal tumor size (< 10/≥ 10 cm) | 1.693 (0.894-3.204) | ||
| Tumor volume (< 400/≥ 400 cm3) | 2.509 (1.289-4.885) | 2.520 (1.252-5.072) | 0.01 |
| Child-Pugh score (5/> 5) | 2.099 (1.038-4.244) | 1.812 (0.878-3.738) | 0.108 |
| Stage | |||
| mUICC stage (III/IV) | 1.198 (0.502-2.863) | ||
| AJCC (III/IV) | 2.133 (1.085-4.193) | 1.803 (0.895-3.634) | 0.099 |
| Extrahepatic metastases | 1.508 (0.772-2.948) | ||
| Pre-HAIC treatment | 0.939 (0.503-1.755) | ||
| Portal vein thrombosis (Vp4 vs non-Vp4) | 1.603 (0.829-3.100) | ||
| AFP level (< 200/≥ 200 ng/mL) | 1.707 (0.845-3.448) | ||
| PIVKA-II (< 40/≥ 40 mAU/mL) | 2.860 (1.110-7.368) | 3.121 (1.177-8.274) | 0.022 |
Those variables with P < 0.05 in univariate analysis were included. AFP: Alpha-fetoprotein; AJCC: American Joint Committee on Cancer; HAIC: Hepatic arterial infusion chemotherapy; PIVKA: Protein induced by vitamin K absence or antagonist; mUICC: Modified Union for International Cancer Control.
Table 4.
Post-treatment prognostic factors for survival in hepatic arterial infusion chemotherapy treatment
| Variables | Univariate | Multivariate1 | ||
| HR (95%CI) | Adjusted HR (95%CI) | P-value2 | ||
| After 2nd HAIC cycle | Objective response | |||
| Responder | 1 | |||
| Non-responder | 2.382 (0.995-5.704) | |||
| Disease control | ||||
| Control group | 1 | |||
| Progressive group | 3.708 (1.801-7.634) | 3.850 (1.768-8.381) | 0.001 | |
| PVTT response | ||||
| Response | 1 | |||
| Non-response | 2.531 (1.164-5.505) | 3.398 (1.529-7.552) | 0.003 | |
| AFP reduction | ||||
| ≥ 50% | 1 | |||
| < 50% | 3.242 (1.297-8.102) | 3.031 (1.194-7.691) | 0.02 | |
| PIVKA-II reduction | ||||
| ≥ 50% | 1 | |||
| < 50% | 3.164 (1.469-6.818) | 2.254 (0.989-5.137) | 0.053 | |
| Response during HAIC | Best tumor response | |||
| Objective response | ||||
| Responder | 1 | |||
| Non-responder | 4.747 (2.111-10.672) | 4.445 (1.893-10.439) | 0.001 | |
| Tumor control | ||||
| Control group | 1 | |||
| Progressive group | 3.274 (1.594-6.724) | 3.137 (1.494-6.591) | 0.003 | |
| Intra-hepatic tumor response | ||||
| Objective response | ||||
| Responder | 1 | |||
| Non-responder | 4.747 (2.111-10.672) | 4.445 (1.893-10.439) | 0.001 | |
| Tumor control | ||||
| Control group | 1 | |||
| Progressive group | 3.032 (1.348-6.821) | 3.009 (1.302-6.958) | 0.01 | |
| PVTT response | ||||
| Response | 1 | |||
| Non-response | 9.587 (2.879-31.927) | 8.188 (2.403-27.898) | 0.001 | |
| Combination therapy | ||||
| Yes | 1 | |||
| No | 2.367 (1.218-4.601) | 2.164 (1.082-4.328) | 0.029 | |
Adjusted for tumor volume and pre-treatment PIVKA-II level;
P value for adjusted hazard ratio. AFP: Alpha-fetoprotein; HAIC: Hepatic arterial infusion chemotherapy; PIVKA: Protein induced by vitamin K absence or antagonist; PVTT: Portal vein tumor thrombosis.
Figure 3.
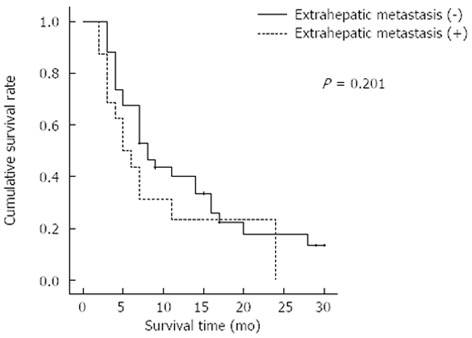
Cumulative survival rate according to the presence of extrahepatic metastasis.
The post-treatment parameters were analyzed after the second cycle and at the end of HAIC. Univariate analysis after the second cycle of HAIC determined that objective response, disease control, portal vein tumor thrombosis response, and ≥ 50% reduction of AFP and PIVKA-II level were significant post-treatment prognostic factors. Multivariate analysis after the second cycle of HAIC determined that disease control [P = 0.001, HR = 3.850 (95%CI: 1.768-8.381)], PVTT response [P = 0.003, HR = 3.398 (95%CI: 1.529-7.552)] and ≥ 50% AFP reduction [P = 0.02, HR = 3.031 (95%CI: 1.194-7.691)] were significant predictors for longer survival. At the end of the HAIC treatment, univariate analysis was performed with combination therapy and factors related to tumor response. Six factors were significant: the overall tumor response (including objective response and disease control), intrahepatic tumor response (including objective response and disease control), PVTT response, and combination therapy. A multivariate analysis at the end of HAIC treatment determined that objective tumor response [P = 0.001, HR = 4.445 (95%CI: 1.893-10.439)], disease control [P = 0.003, HR = 3.137 (95%CI: 1.494-6.591)], objective intrahepatic tumor response [P = 0.001, HR = 4.445 (95%CI: 1.893-10.439)], intrahepatic tumor control [P = 0.01, HR = 3.009 (95%CI: 1.302-6.958)], PVTT response [P = 0.001, HR = 8.188 (95%CI: 2.403-27.898)], and combination therapy [P = 0.029, HR = 2.164 (95%CI: 1.082-4.328)] were independent predictors for longer survival.
Toxicity
The toxicities observed in this study are summarized in Table 5. No treatment-related mortality was detected. The most common toxicities were anemia and aspartate aminotransferase (AST) elevation, and all patients showed a toxicity grade of at least 1. The most common grade 3/4 toxicities were thrombocytopenia and AST elevation (22 patients, 44%). The toxicities were transient, tolerable, and successfully managed using a conservative treatment; there were no discontinuation of the treatment due to toxicity. Hepatic arterial thrombosis developed in 4 of the 50 patients. However, thrombolysis by urokinase was effectively performed, and port removal was required in only 1 patient. Overall, 37 (74%) of the 50 patients in the treatment group died during the follow-up. The causes of death are listed in Table 6. The most common cause of death was tumor progression (57%), and six patients (16%) died from deteriorating hepatic function without any evidence of tumor progression, sepsis, or gastrointestinal bleeding. Five patients (14%) died from variceal bleeding, and three (8%) died from infection. Ten patients (20%) were still alive when the final analysis was performed, and three patients (6%) were lost to follow-up.
Table 5.
Adverse events related to treatment
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Hematological | ||||
| Leukopenia | 26% | 38% | 8% | 0% |
| Neutropenia | 10% | 26% | 30% | 12% |
| Anemia | 26% | 48% | 26% | 0% |
| Thrombocytopenia | 30% | 18% | 44% | 0% |
| Non-hematological | ||||
| Total bilirubin | 30% | 28% | 10% | 0% |
| AST | 20% | 36% | 38% | 6% |
| ALT | 48% | 12% | 16% | 2% |
ALT: Alanine aminotransferase; AST: Aspartate aminotransferase.
Table 6.
Causes of death
| Patient number (n = 37) | |
| Disease progression | 21 |
| Hepatic dysfunction | 6 |
| Variceal bleeding | 5 |
| Infection | 3 |
| Unknown | 2 |
DISCUSSION
Portal vein tumor invasion is a common complication in HCC, reportedly observed in 64.7% of cases at autopsy[20]. PVTT often leads to extensive spreading of the tumor and can increase portal venous blood pressure, resulting in the fatal rupture of esophageal varices. PVTT can also decrease portal flow that may lead to ascites, jaundice, hepatic encephalopathy, or liver failure. Therefore, the presence of PVTT is one of the most significant prognostic factors of poor prognosis[21,22], and it has been reported that these patients survive only 2.7-4 mo if left untreated[22,23]. In advanced HCC patients with PVTT, standard treatments have not been established, especially in the Asia-Pacific region. Though the BCLC staging system recommends sorafenib in these patients, its efficacy is limited. Thus, HAIC is considered an alternative treatment modality, especially in Japan and South Korea.
In this study, we analyzed the response rate and overall survival of HAIC using the ECF regimen. In previous reports, the response rate and disease control rate of HAIC in advanced HCC patients with PVTT[11,24-26] were 33%-52% and 47%-77%, respectively. The median OS was 7-10 mo in those studies. The objective response rate and disease control rate of our study, which are 32% and 76%, respectively, are in accordance with the above results. In addition, the median OS of 7 mo was similar to the results of previous reports. However, our study included patients with extrahepatic metastasis at the beginning of HAIC, whereas the previous studies excluded those patients. HAIC treatment is primarily used for the local control of liver tumors in patients with minimal extrahepatic spread[7]. Extrahepatic tumors would not respond well to HAIC. However, our data support the use of HAIC in HCC patients with extrahepatic metastasis because extrahepatic metastasis was not a significant factor in survival (Figure 3). The mortality in advanced HCC is related to intrahepatic tumors, and the leading cause of death in these patients is intrahepatic tumor progression[27,28]. In this study, all patients had advanced intrahepatic HCC with vascular invasion. Because the survival of these patients was influenced by intrahepatic tumor progression, extrahepatic metastasis may not influence overall survival. If the treatment response evaluation is confined to intrahepatic tumor lesions, the disease control rate was as high as 84%. These response and disease control rates are significantly higher than those with sorafenib treatment[5,29].
Several studies reported that the therapeutic effectiveness of HAIC was an important prognostic factor[10,11,30], which is consistent with our results. In the present study, the median OS of the disease control group after two cycles of HAIC was significantly longer than patients showing PD. In addition, patients with an objective response to HAIC treatment also had significantly longer survival than non-responders. These results indicate that the responses to HAIC were independent prognostic factors. In addition, this study showed that the response after the second cycle of HAIC also significantly influences survival; thus, patients with CR, PR or SD after the second cycle of HAIC could continue HAIC treatment and expect favorable results.
PIVKA-II, also known as a des-gamma carboxy prothrombin (DCP), is an alternative tumor marker of AFP in diagnosing HCC. It is associated with aggressive features such as tumor size, vascular invasion, tumor stage and survival, and patients with high serum PIVKA-II levels have a poor prognosis[31,32]. In the present study, the median OS in patients with a PIVKA-II level ≥ 40 and < 40 mAU/mL were 7 and 16 mo, respectively (Figure 4). Thus, patients with high serum PIVKA-II levels prior to HAIC treatment would be expected to have a poorer prognosis. Some reports state that, independent of pre-treatment level, tumor marker response to treatment was associated with survival. Park et al[18] reported that AFP and DCP response were independent factors associated with survival. Personeni et al[17] reported that AFP response is an independent surrogate end point for survival in patients treated with sorafenib. Similarly, a ≥ 50% decline in AFP and PIVKA-II after the second cycle of HAIC treatment was associated with better outcomes among the patients with elevated AFP and PIVKA-II levels prior to the initiation of HAIC treatment (though PIVKA-II reduction is not statistically significant by multivariate analysis) (Figure 4). Therefore, tumor marker response (AFP and PIVKA-II) after the second cycle may be a useful surrogate endpoint for good outcomes in those receiving HAIC treatment for large HCC with PVTT.
Figure 4.
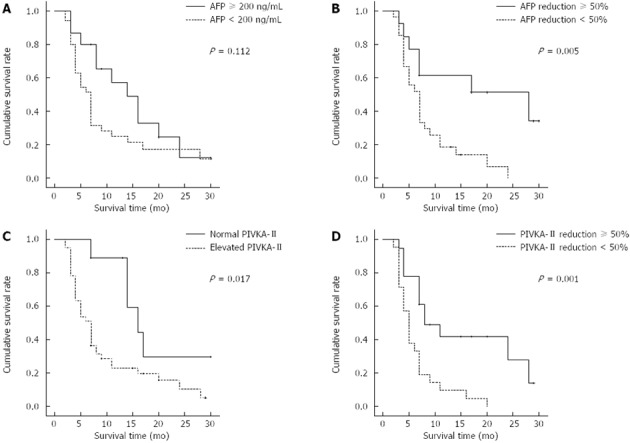
Cumulative survival rates. A: According to the pre-treatment α-fetoprotein (AFP) level; B: According to the AFP reduction after two cycles of hepatic arterial infusion chemotherapy (HAIC); C: According to the pre-treatment protein induced by vitamin K absence or antagonist (PIVKA)-II level; D: According to the PIVKA-II reduction after two cycles of HAIC.
Many studies have shown that tumor size is a major determinant of survival[33,34]. However, few studies (with the exception of Hsu et al) have analyzed survival according to tumor volume[35]. In the current study, multivariate analysis of the pre-treatment parameters showed that a tumor volume < 400 cm3 was an independent pre-treatment prognostic factor, while the maximal tumor diameter was not. Along with tumor volume, we evaluated the Child-Pugh score as a predictive factor of survival. Though not a significant multivariate variable, a Child-Pugh score of 5 was an important factor predicting good outcome (median OS of Child class A5, A6 and B were 16, 7 and 3 mo, respectively). This result is consistent with the results of HAIC studies showing that the Child-Pugh score was an independent survival factor[30,36]. Thus, HAIC using ECF could be indicated in selected patients with tumor volumes < 400 cm3, good hepatic reserve function, and low PIVKA-II levels.
Although many studies using HAIC have been performed over the last decade, the therapeutic regimen of this treatment has not been standardized. Ando et al[11] reported that HAIC using low-dose cisplatin and 5-FU demonstrated a good response rate and survival time in 48 patients with PVTT. Park et al[10] showed that repetitive HAIC with high-dose 5-FU and cisplatin given for 3 d was effective and safe. In Japan, interferon-combined HAIC is also commonly used. The ECF chemotherapeutic regimen in this study consisted of high dose cisplatin and 5-FU in combination with epirubicin. Woo et al[14] compared high-dose HAIC with low-dose HAIC and reported that high-dose HAIC was safe and achieved better tumor response compared with that of low-dose HAIC. The addition of epirubicin to the high-dose HAIC regimen resulted in more effective control of the intrahepatic tumor in our study.
However, the ECF regimen appears to be more toxic than in previous reports of high-dose HAIC[10,14]. While grade 3 hematologic or non-hematologic toxicities comprised less than 5% in previous studies, grade 3 leukopenia, anemia, and thrombocytopenia in this study comprised 8%, 26% and 44% of the toxicities, respectively, and hepatic toxicity was higher. In addition, hematologic toxicities tended to be more common in this study than in the trials using sorafenib such as the SHARP trial or an Asia-Pacific trial[5,29]. The more frequent grade 3/4 toxicities in this study may be due to our use of different inclusion criteria. The hematologic inclusion criteria were lower than those used in the previous study, and there were no inclusion criteria related to hepatic function such as AST, ALT and bilirubin. As a result, our study included more patients with lower blood cell counts or higher liver enzyme levels, which could lead to more toxic adverse events. However, all hematologic and hepatic toxicity returned to baseline levels within several days.
This study has some limitations. First, we included patients with extrahepatic metastasis even though HAIC is only considered effective for the treatment of intrahepatic tumors[7]. However, extrahepatic metastasis was not independently associated with survival, and the results were as good as those of previous studies despite the inclusion of patients with extrahepatic metastasis. Second, because most tumors were of the infiltrative type and margins were obscure, there was difficulty in accurately measuring tumor volume. Third, the retrospective nature of this study is underpowered due to a single arm registry without a control group. In addition, this study may have inherent bias associated with a small sample size and heterogeneous treatments. Thus, large prospective studies are necessary to establish the efficacy of HAIC using the ECF regimen in patients with large tumors and PVTT.
In conclusion, HAIC with the ECF regimen may be a good option for advanced HCC with tumor volumes < 400 cm3 and a normal PIVKA-II level. In addition, this study suggests that response to chemotherapy after two cycles of HAIC, including radiologic tumor control and tumor marker reduction, is an independent predictor of longer survival. Therefore, HAIC treatment could be indicated in selected patients with favorable pre-treatment or post-treatment prognostic factors.
COMMENTS
Background
Hepatocellular carcinoma (HCC) is a major health problem, accounting for more than 626000 new cases per year worldwide. Most HCC patients are diagnosed at an inoperable (intermediate or advanced) stage. The prognosis for advanced HCC is dismal. Sorafenib is the only approved drug for target therapy, and this drug is recommended for advanced HCC patients according to Barcelona Clinic Liver Cancer staging system. However, sorafenib shows only modest improvements in survival and tumor response.
Research frontiers
Hepatic arterial infusion chemotherapy (HAIC) has been used as a treatment modality for advanced HCC. Although sorafenib is regarded as the standard treatment of advanced HCC, HAIC can be an alternative treatment modality. The research hotspot in the area of HAIC treatment involves patients who will have good responses to HAIC.
Innovations and breakthroughs
Until now, the HAIC therapeutic regimen has not been standardized. This study is the first to use the epirubicin-cisplatin-5-fluorouracil (ECF) regimen, which consists of epirubicin, cisplatin, and 5-fluorouracil. HAIC treatment using the ECF regimen showed a good response, and its toxicity was tolerable. While previous studies on HAIC attempted to investigate the prognostic factors among the pre-treatment variables and the best response during treatment, this study revealed that the response after the second cycle of treatment, including radiologic response and tumor marker reduction, is also an important prognostic factor. The authors measured the actual tumor volume using imaging software and suggested a cut-off volume that could indicate a good response to HAIC.
Applications
Because the response after the second cycle is an important prognostic factor, those who showed a good response after the second cycle can continue the HAIC treatment and can expect favorable results.
Terminology
HAIC: HAIC is one type of arterial chemotherapy used in advanced hepatocellular carcinoma. HAIC requires an implantable port system for which a catheter is inserted and localized to the proper hepatic artery. HAIC enables the local delivery of high concentrations of anti-cancer agents to tumors, thereby maintaining a low systemic concentration of chemotherapeutic agent.
Peer review
This is a good article about the use of HAIC in the treatment of advanced HCC with portal vein tumor thrombosis. HAIC is shown to be an effective treatment modality.
Footnotes
Supported by National R and D Program Grant for Cancer Control from the Ministry of Health, Welfare and Family Affairs, Republic of Korea (R0620390-1)
P- Reviewers Chan ACY, Cong WM, Kagawa T, Lai Q, Ramani K, Tao P S- Editor Wen LL L- Editor A E- Editor Zhang DN
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Sangiovanni A, Del Ninno E, Fasani P, De Fazio C, Ronchi G, Romeo R, Morabito A, De Franchis R, Colombo M. Increased survival of cirrhotic patients with a hepatocellular carcinoma detected during surveillance. Gastroenterology. 2004;126:1005–1014. doi: 10.1053/j.gastro.2003.12.049. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 4.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 6.Cheng AL, Guan Z, Chen Z, Tsao CJ, Qin S, Kim JS, Yang TS, Tak WY, Pan H, Yu S, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma according to baseline status: subset analyses of the phase III Sorafenib Asia-Pacific trial. Eur J Cancer. 2012;48:1452–1465. doi: 10.1016/j.ejca.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Shao YY, Huang CC, Liang PC, Lin ZZ. Hepatic arterial infusion of chemotherapy for advanced hepatocellular carcinoma. Asia Pac J Clin Oncol. 2010;6:80–88. doi: 10.1111/j.1743-7563.2010.01287.x. [DOI] [PubMed] [Google Scholar]
- 8.Okada S, Okazaki N, Nose H, Yoshimori M, Aoki K. Prognostic factors in patients with hepatocellular carcinoma receiving systemic chemotherapy. Hepatology. 1992;16:112–117. doi: 10.1002/hep.1840160119. [DOI] [PubMed] [Google Scholar]
- 9.Nerenstone SR, Ihde DC, Friedman MA. Clinical trials in primary hepatocellular carcinoma: current status and future directions. Cancer Treat Rev. 1988;15:1–31. doi: 10.1016/0305-7372(88)90007-2. [DOI] [PubMed] [Google Scholar]
- 10.Park JY, Ahn SH, Yoon YJ, Kim JK, Lee HW, Lee do Y, Chon CY, Moon YM, Han KH. Repetitive short-course hepatic arterial infusion chemotherapy with high-dose 5-fluorouracil and cisplatin in patients with advanced hepatocellular carcinoma. Cancer. 2007;110:129–137. doi: 10.1002/cncr.22759. [DOI] [PubMed] [Google Scholar]
- 11.Ando E, Tanaka M, Yamashita F, Kuromatsu R, Yutani S, Fukumori K, Sumie S, Yano Y, Okuda K, Sata M. Hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis: analysis of 48 cases. Cancer. 2002;95:588–595. doi: 10.1002/cncr.10694. [DOI] [PubMed] [Google Scholar]
- 12.Kim HY, Kim JD, Bae SH, Park JY, Han KH, Woo HY, Choi JY, Yoon SK, Jang BK, Hwang JS, et al. A comparative study of high-dose hepatic arterial infusion chemotherapy and transarterial chemoembolization using doxorubicin for intractable, advanced hepatocellular carcinoma. Korean J Hepatol. 2010;16:355–361. doi: 10.3350/kjhep.2010.16.4.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eun JR, Lee HJ, Moon HJ, Kim TN, Kim JW, Chang JC. Hepatic arterial infusion chemotherapy using high-dose 5-fluorouracil and cisplatin with or without interferon-alpha for the treatment of advanced hepatocellular carcinoma with portal vein tumor thrombosis. Scand J Gastroenterol. 2009;44:1477–1486. doi: 10.3109/00365520903367262. [DOI] [PubMed] [Google Scholar]
- 14.Woo HY, Bae SH, Park JY, Han KH, Chun HJ, Choi BG, Im HU, Choi JY, Yoon SK, Cheong JY, et al. A randomized comparative study of high-dose and low-dose hepatic arterial infusion chemotherapy for intractable, advanced hepatocellular carcinoma. Cancer Chemother Pharmacol. 2010;65:373–382. doi: 10.1007/s00280-009-1126-2. [DOI] [PubMed] [Google Scholar]
- 15.Korean Liver Cancer Study Group and National Cancer Center, Korea. [Practice guidelines for management of hepatocellular carcinoma 2009] Korean J Hepatol. 2009;15:391–423. doi: 10.3350/kjhep.2009.15.3.391. [DOI] [PubMed] [Google Scholar]
- 16.Song do S, Bae SH. Changes of guidelines diagnosing hepatocellular carcinoma during the last ten-year period. Clin Mol Hepatol. 2012;18:258–267. doi: 10.3350/cmh.2012.18.3.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Personeni N, Bozzarelli S, Pressiani T, Rimassa L, Tronconi MC, Sclafani F, Carnaghi C, Pedicini V, Giordano L, Santoro A. Usefulness of alpha-fetoprotein response in patients treated with sorafenib for advanced hepatocellular carcinoma. J Hepatol. 2012;57:101–107. doi: 10.1016/j.jhep.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 18.Park WH, Shim JH, Han SB, Won HJ, Shin YM, Kim KM, Lim YS, Lee HC. Clinical utility of des-γ-carboxyprothrombin kinetics as a complement to radiologic response in patients with hepatocellular carcinoma undergoing transarterial chemoembolization. J Vasc Interv Radiol. 2012;23:927–936. doi: 10.1016/j.jvir.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 19. Available from: http://ctep.cancer.gov.
- 20.Nakashima T, Okuda K, Kojiro M, Jimi A, Yamaguchi R, Sakamoto K, Ikari T. Pathology of hepatocellular carcinoma in Japan. 232 Consecutive cases autopsied in ten years. Cancer. 1983;51:863–877. doi: 10.1002/1097-0142(19830301)51:5<863::aid-cncr2820510520>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 21.Park KW, Park JW, Choi JI, Kim TH, Kim SH, Park HS, Lee WJ, Park SJ, Hong EK, Kim CM. Survival analysis of 904 patients with hepatocellular carcinoma in a hepatitis B virus-endemic area. J Gastroenterol Hepatol. 2008;23:467–473. doi: 10.1111/j.1440-1746.2007.05112.x. [DOI] [PubMed] [Google Scholar]
- 22.Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, Nakajima Y, Ohnishi K. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918–928. doi: 10.1002/1097-0142(19850815)56:4<918::aid-cncr2820560437>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 23.Villa E, Moles A, Ferretti I, Buttafoco P, Grottola A, Del Buono M, De Santis M, Manenti F. Natural history of inoperable hepatocellular carcinoma: estrogen receptors’ status in the tumor is the strongest prognostic factor for survival. Hepatology. 2000;32:233–238. doi: 10.1053/jhep.2000.9603. [DOI] [PubMed] [Google Scholar]
- 24.Nagano H, Wada H, Kobayashi S, Marubashi S, Eguchi H, Tanemura M, Tomimaru Y, Osuga K, Umeshita K, Doki Y, et al. Long-term outcome of combined interferon-α and 5-fluorouracil treatment for advanced hepatocellular carcinoma with major portal vein thrombosis. Oncology. 2011;80:63–69. doi: 10.1159/000328281. [DOI] [PubMed] [Google Scholar]
- 25.Obi S, Yoshida H, Toune R, Unuma T, Kanda M, Sato S, Tateishi R, Teratani T, Shiina S, Omata M. Combination therapy of intraarterial 5-fluorouracil and systemic interferon-alpha for advanced hepatocellular carcinoma with portal venous invasion. Cancer. 2006;106:1990–1997. doi: 10.1002/cncr.21832. [DOI] [PubMed] [Google Scholar]
- 26.Lai YC, Shih CY, Jeng CM, Yang SS, Hu JT, Sung YC, Liu HT, Hou SM, Wu CH, Chen TK. Hepatic arterial infusion chemotherapy for hepatocellular carcinoma with portal vein tumor thrombosis. World J Gastroenterol. 2003;9:2666–2670. doi: 10.3748/wjg.v9.i12.2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jung SM, Jang JW, You CR, Yoo SH, Kwon JH, Bae SH, Choi JY, Yoon SK, Chung KW, Kay CS, et al. Role of intrahepatic tumor control in the prognosis of patients with hepatocellular carcinoma and extrahepatic metastases. J Gastroenterol Hepatol. 2012;27:684–689. doi: 10.1111/j.1440-1746.2011.06917.x. [DOI] [PubMed] [Google Scholar]
- 28.Uka K, Aikata H, Takaki S, Shirakawa H, Jeong SC, Yamashina K, Hiramatsu A, Kodama H, Takahashi S, Chayama K. Clinical features and prognosis of patients with extrahepatic metastases from hepatocellular carcinoma. World J Gastroenterol. 2007;13:414–420. doi: 10.3748/wjg.v13.i3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 30.Kirikoshi H, Yoneda M, Mawatari H, Fujita K, Imajo K, Kato S, Suzuki K, Kobayashi N, Kubota K, Maeda S, et al. Is hepatic arterial infusion chemotherapy effective treatment for advanced hepatocellular carcinoma resistant to transarterial chemoembolization? World J Gastroenterol. 2012;18:1933–1939. doi: 10.3748/wjg.v18.i16.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim HS, Park JW, Jang JS, Kim HJ, Shin WG, Kim KH, Lee JH, Kim HY, Jang MK. Prognostic values of alpha-fetoprotein and protein induced by vitamin K absence or antagonist-II in hepatitis B virus-related hepatocellular carcinoma: a prospective study. J Clin Gastroenterol. 2009;43:482–488. doi: 10.1097/MCG.0b013e318182015a. [DOI] [PubMed] [Google Scholar]
- 32.Kang SH, Kim do Y, Jeon SM, Ahn SH, Park JY, Kim SU, Kim JK, Lee KS, Chon CY, Han KH. Clinical characteristics and prognosis of hepatocellular carcinoma with different sets of serum AFP and PIVKA-II levels. Eur J Gastroenterol Hepatol. 2012;24:849–856. doi: 10.1097/MEG.0b013e3283535c34. [DOI] [PubMed] [Google Scholar]
- 33.Takayasu K, Arii S, Ikai I, Omata M, Okita K, Ichida T, Matsuyama Y, Nakanuma Y, Kojiro M, Makuuchi M, et al. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology. 2006;131:461–469. doi: 10.1053/j.gastro.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 34.Murakami E, Aikata H, Miyaki D, Nagaoki Y, Katamura Y, Kawaoka T, Takaki S, Hiramatsu A, Waki K, Takahashi S, et al. Hepatic arterial infusion chemotherapy using 5-fluorouracil and systemic interferon-α for advanced hepatocellular carcinoma in combination with or without three-dimensional conformal radiotherapy to venous tumor thrombosis in hepatic vein or inferior vena cava. Hepatol Res. 2012;42:442–453. doi: 10.1111/j.1872-034X.2011.00943.x. [DOI] [PubMed] [Google Scholar]
- 35.Hsu CY, Huang YH, Hsia CY, Su CW, Lin HC, Loong CC, Chiou YY, Chiang JH, Lee PC, Huo TI, et al. A new prognostic model for hepatocellular carcinoma based on total tumor volume: the Taipei Integrated Scoring System. J Hepatol. 2010;53:108–117. doi: 10.1016/j.jhep.2010.01.038. [DOI] [PubMed] [Google Scholar]
- 36.Baek YH, Kim KT, Lee SW, Jeong JS, Park BH, Nam KJ, Cho JH, Kim YH, Roh YH, Lee HS, et al. Efficacy of hepatic arterial infusion chemotherapy in advanced hepatocellular carcinoma. World J Gastroenterol. 2012;18:3426–3434. doi: 10.3748/wjg.v18.i26.3426. [DOI] [PMC free article] [PubMed] [Google Scholar]


