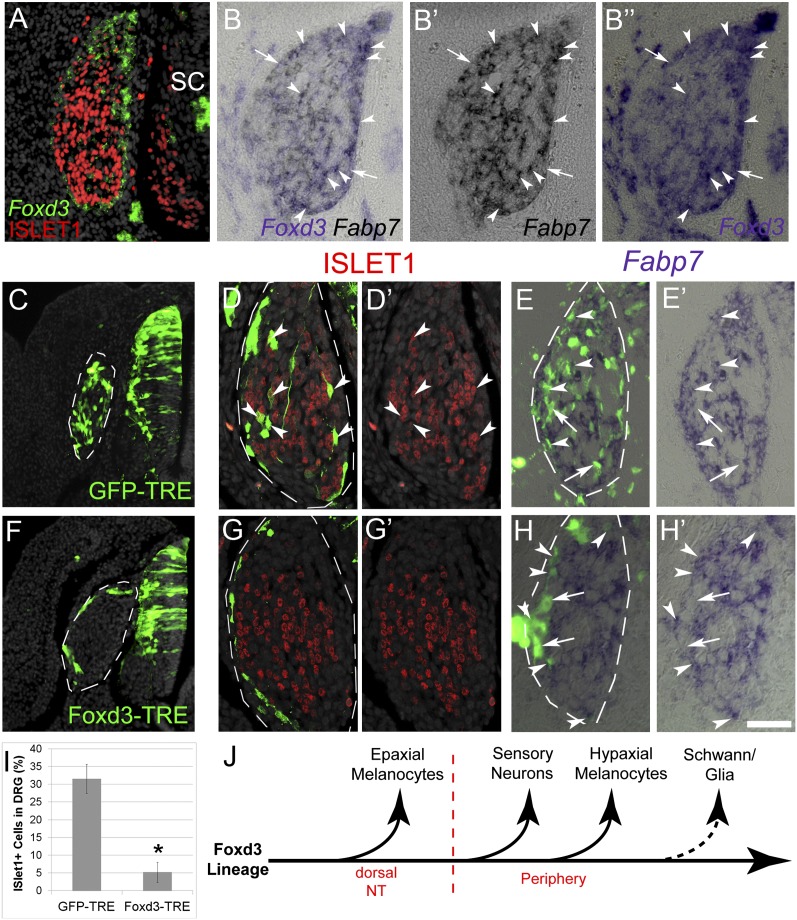
Fig. 5.
Sustained Foxd3 activity in DRG inhibits neuronal development. (A) Double ISH/immunostaining for Foxd3 and ISLET-1, respectively, on E5 DRG showing an exclusive pattern of marker expression. (B–B’’) ISH for Foxd3 and Fabp7 on adjacent 6-µm sections of E5 DRG. Arrowheads mark double-positive cells, and arrows point to Foxd3+/Fabp7- cells. (C–I) Conditional misexpression of Foxd3 in DRG. pCAGGS-rtTA2s-M2 together with either pBI-TRE-GFP or pBI-TRE-Foxd3 were electroporated into hemitubes at E2. Doxycyclin was added 32 h after electroporation when NC cells already formed DRG, and analysis was performed 24 h later. (C and F) Note homogeneous localization of control GFP+ cells in DRG (C, D, and E) contrasting with the distribution of Foxd3/GFP+ cells to the ganglion periphery (F, G, and H). (D–E’ and G–H’) Costaining of GFP with ISLET1 (D and G) or Fabp7 (E and H). Arrowheads point to double-positive neurons or glia and arrows to GFP+/Fabp7- progenitors. (I) Quantification of ISLET+ of total GFP+ cells. *P < 0.005. (J) Scheme representing the progressive segregation of NC derivatives from the Foxd3 lineage, based on data from Nitzan et al. (8) and the present study. SC/glia are the last to lose Foxd3 expression upon differentiation (dotted arrow). SC, spinal cord. (Scale bar, 30 μm in A–B”; 50 μm in C and F; 25 μm in D–E’ and G–H’.)