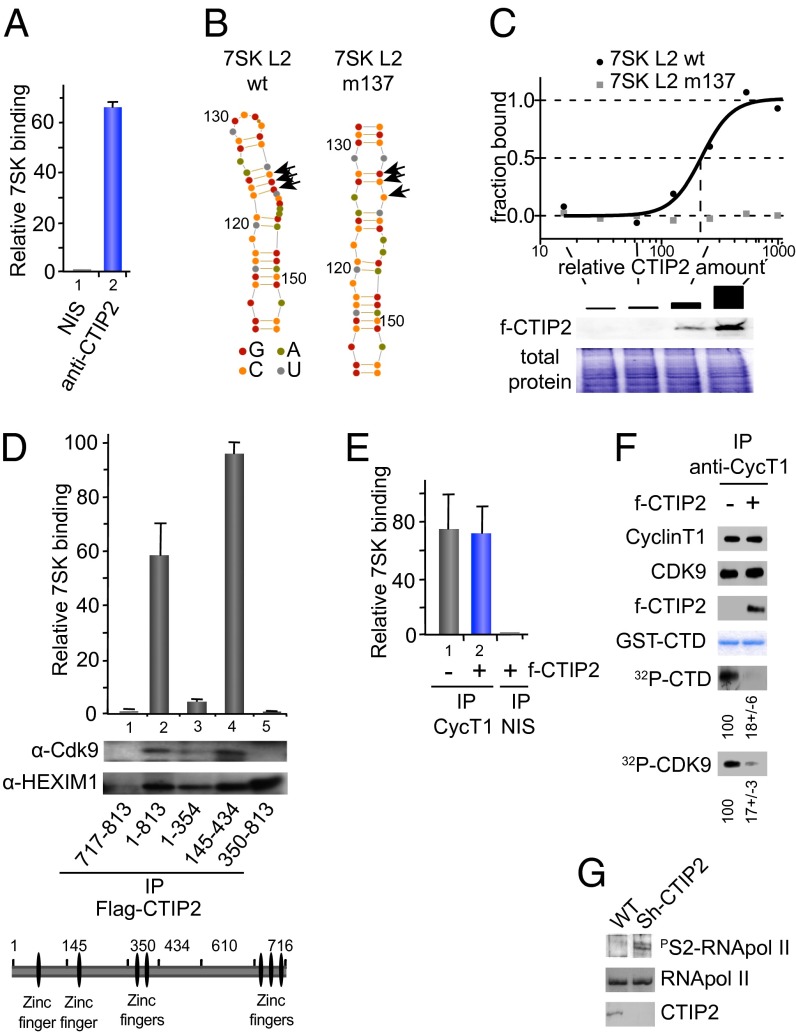
Fig. 2.
Associated with the 7SK snRNA, CTIP2 inhibits Cdk9-mediated phosphorylation. (A) HEK293T cells were lysed and subjected to immunoprecipitations with indicated antibodies. The presence of 7SK snRNA was quantified by RT-Q-PCR after complex elution and RNA extraction. (B) Secondary structures of 7SK L2 and the mutant 7SK L2 m137 were calculated using Assemble software (version 2.1); nucleotides are indicated by color code and numbered with respect to their position in full-length 7SK RNA and arrows indicate mutations in m137. (C) MST analyses were performed with cellular extracts containing increasing amounts of FLAG-CTIP2 and Fam6-labeled 7SK L2 and 7SK L2 m137. The FLAG-CTIP2 amount was monitored by Western blot using anti-FLAG antibody, and the total protein amount was visualized by Coomassie staining. The highest CTIP2 concentration was abitrarily set to 1,000. (D) Mock, FLAG-CTIP2 wild-type, or FLAG-CTIP2 constructs transfected HEK293T cells were lysed and subjected to immunoprecipitation with anti-FLAG antibody. The presence of 7SK snRNA was quantified by RT-Q-PCR after complex elution and RNA extraction. Detection of immunoprecipitated proteins was determined by Western blot analysis with the indicated antibodies. (E) Mock or FLAG-CTIP2 transfected cells were lysed and subjected to immunoprecipitations with the indicated antibodies. The presence of 7SK snRNA was quantified by RT-Q-PCR after complex elution and RNA extraction. (F) FLAG-CTIP2 or mock transfected cells were lysed and subjected to immunoprecipitation with anti-CyclinT1 antibody. Immunoprecipitated proteins were incubated for 1 h at 30 °C with GST-CTD and 1 μCi of 32P-labeled ATP. After SDS/PAGE, labeled GST-CTD and endogenous Cdk9 were detected by autoradiography. Quantification of the signals was performed using ImageJ software. The presence of the indicated proteins was determined by Western blot analysis with the indicated antibodies. The amount of GST-CTD was assessed by Coomassie staining. Specificity for the GST-CTD substrate was controlled by using mutated GST-CTD (Fig. S5). (G) Extracts from WT or CTIP2 knockdown cell lines were subjected to Western blot analysis with the indicated antibodies.