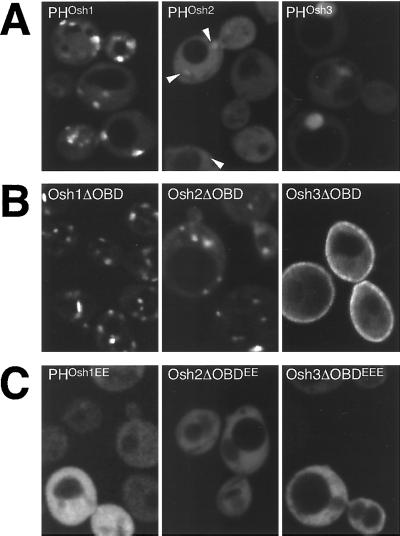
Figure 8.
Targeting by the three Osh PH domains. GFP-tagged portions of Osh1-3p visualized in log phase Δosh1 cells (TLY221). (A) PH domains of Osh proteins have different targeting strengths. PHOsh1 localizes readily to punctate structures, PHOsh2 is largely diffuse, except for a number of barely detectable punctate structures seen against the strong cytoplasmic background (arrowheads) and PHOsh3 is diffuse with some nuclear localization. Plasmids pTL331, pTL342, pTL343. (B) Constructs containing all but the oxysterol binding domains target to distinct intracellular localizations (plasmids pTL321, pTL352, pTL353). Compared with the PH domain alone, Osh1ΔOBD shows additional NV junction localization and reduced cytoplasmic background, Osh2ΔOBD shows localization to punctate structures, and Osh3ΔOBD shows some localization to the plasma membrane. Osh3ΔOBD shows no nuclear localization, probably because of its increased size. (C) Constructs as in A or B but with conserved basic residues of the variable loop 1 of the PH domain replaced with glutamate residues (plasmids pTL356, pTL357, pTL358). The levels of wild-type and mutant proteins were indistinguishable by protein blotting with anti-GFP antibodies (our unpublished results).