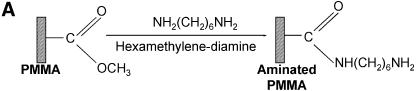
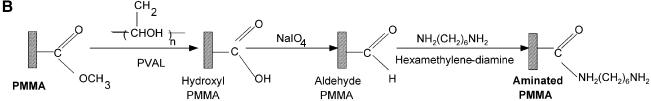
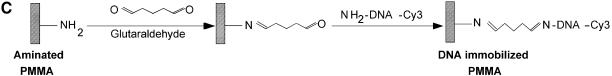
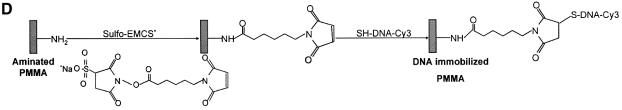
Figure 1.
Chemistries used to modify the PMMA substrates and to covalently immobilize 5′-end modified DNA oligonucleotides to aminated terminated surfaces. (A) The available methyl esters of the PMMA, under basic pH conditions, are reacted with an electron donor (N) present on the hexamethylene- diamine, yielding primary amines on the surface. (B) Protocol described by Bulmus et al. (26). (C) Attaching aminated DNA to aminated PMMA surfaces. The aminated PMMA was treated with the homobifunctional cross-linker glutaraldehyde, having a terminal aldehyde group reacting to the primary amino groups of the PMMA, through an imine bond. The NH2-DNA probes are subsequently reacted with the other aldehyde terminal of the cross-linker, establishing a covalent bond between the surface and the oligonucleotide probe. (D) Attaching thiolated DNA to aminated PMMA surfaces. The aminated PMMA is reacted with the NHS ester group of the heterobifunctional cross-linker sulfo-EMCS and subsequently the maleimide portion of the sulfo-EMCS is reacted with 5′-end thiolated DNA probe, to achieve a covalent immobilized DNA.