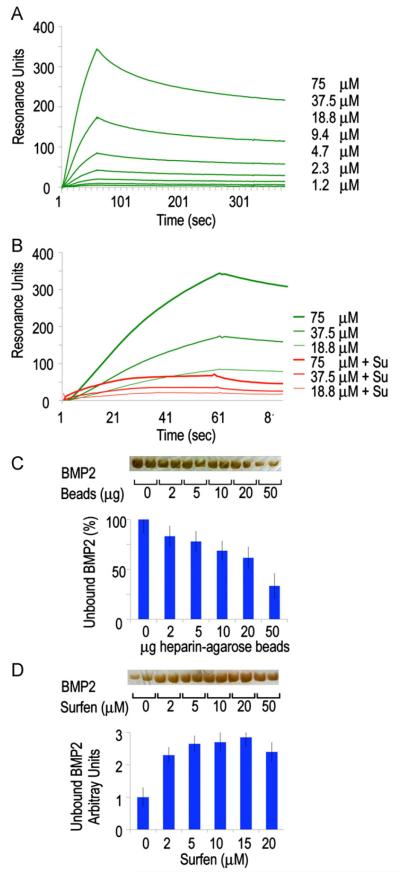
Fig. 7.
Binding interactions between BMP2 and heparin are reversed by HS antagonism. (A) Association–dissociation curves of rhBMP2 injected on a streptavidin chip coated with biotinylated heparin. Binding constants were calculated and are shown in Table 1. (B) Binding of rhBMP2 is substantially decreased when the heparin is pre-treated with Surfen (red lines). (C and D) Solid phase assays (bottom histograms) and gel electrophoresis analysis (top gel bands) showing that the fraction of unbound rhBMP2 decreases with increasing amounts of heparin-coated beads (C), while this trend is reversed in the presence of increasing doses of Surfen (D). Values are means±S.E. of four wells.