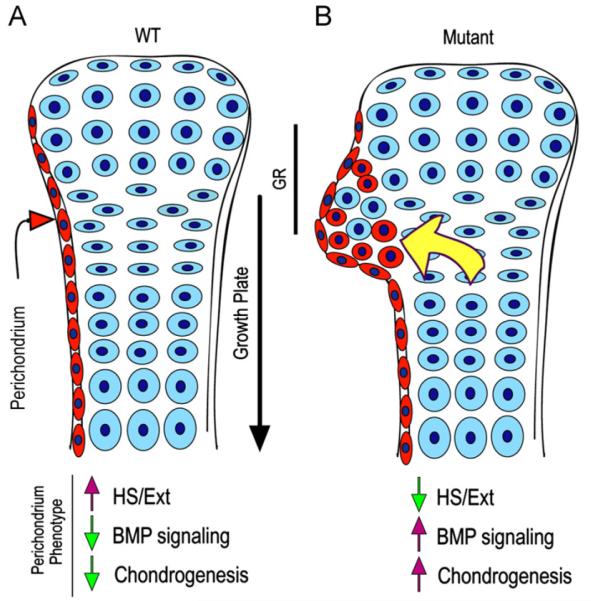
Fig. 8.
Model of ectopic cartilage/exostosis formation. (A) In WT developing long bones, perichondrium (in red) and cartilage (in blue) acquire and maintain their distinct phenotypes and would interact in a variety of ways to sustain skeletal growth and morphogenesis. Normal phenotypic traits of perichondrium would include Ext/HS expression, low protein signaling (such as BMP signaling) and low chondrogenic activity. (B) In conditional Ext mutants (or following treatment with Surfen or heparitinase I), perichondrium would lose its normal phenotypic properties and become depleted of Ext/HS, but would exhibit higher signaling protein and chondrogenic activities. These changes would induce progenitor cells, including those from the groove of Ranvier (GR), to undergo chondrogenesis and form local ectopic cartilage/exostosis (red round cells). The outgrowth process could be aided by recruitment of wild type growth plate cells and also by increased diffusion of BMPs, hedgehogs and growth plate-derived factors (yellow arrow) as we suggested previously (Koyama et al., 2007). Similar overall mechanisms could operate when conditional Ext ablation is directed to growth plate chondrocytes as seen in recent studies (see text). Exostosis formation in HME patients could follow a similar pathogenic cascade or may be more complex given that their EXT mutations are systemic.