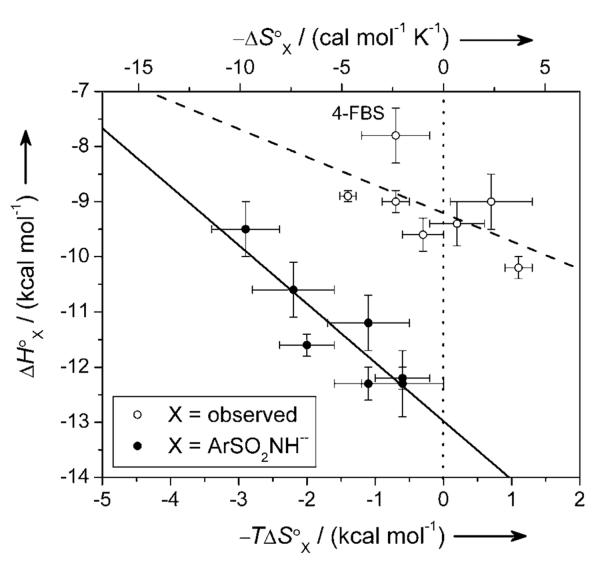
Figure 5.
An enthalpy/entropy compensation plot for the binding of fluorinated benzenesulfonamides to BCA. Error bars are uncertainties described in Table 2. The solid and dashed lines are linear fits to the data. For X = ArSO2NH− (closed circles), the best-fit line gave a value for compensation (negative of the slope) of 1.1 ± 0.2, and a value of R2 of 0.88. For X = observed (the experimentally observed data; open circles), the best-fit line gave a value for compensation (negative of the slope) of 0.5 ± 0.3, and a value of R2 of 0.36; the fit improved only marginally when 4-FBS was excluded from the analysis (R2 = 0.45). The poorer fit to the observed data (X = observed) than to the data calculated for the idealized reaction [X = ArSO2NH−; Eqs. (1) and (5)] illustrates the difficulty of rationalizing the thermodynamics for processes that have a number of steps (e.g., ionization of arylsulfonamide and CA, and binding) without first disentangling the thermodynamics of the individual steps. Uncertainties were given by the linear least-squares fitting procedure. The dotted vertical line separates favorable (−TΔS° < 0) from unfavorable (−TΔS° > 0) entropy of binding.