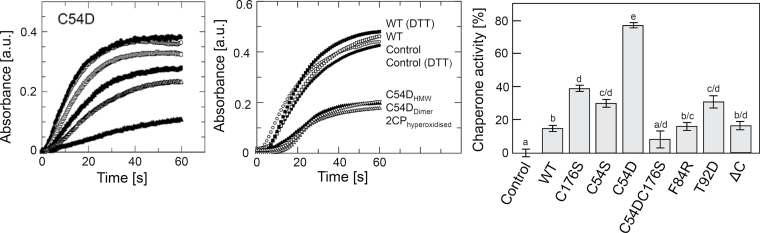
Fig. 6.
Chaperone activity. The light scattering due to thermal aggregation at 45 °C of citrate synthase (1 μM) was visualized by using a spectrophotometer at 360nm. Left panel: chaperone activity of 2-CysPrx C54D in 40mM potassium phosphate buffer (pH 7.2) at the concentrations of 0 (●), 0.5 (◯), 1 (□), 2 (■), 4 (△) and 10 (▲) μM. Middle panel: chaperone activity of oxidized WT (□), reduced WT (■), hyperoxidized WT (△) and non-treated C54D variant at concentrations of 2 μM in 50mM HEPES buffer (pH 7.0). C54D from fractions eluted by size-exclusion chromatography corresponded to a decamer (▽) or lower molecular mass (▲). The controls contained no 2-CysPrx (◯) or 10mM DTT (●). Right panel: chaperone activities of variants at 10 μM in 40mM potassium phosphate buffer (pH 7.2). Data are means ±standard error (n=10). Different letters indicate the significance of groups at P ≤0.05 determined using Student’s t-test.