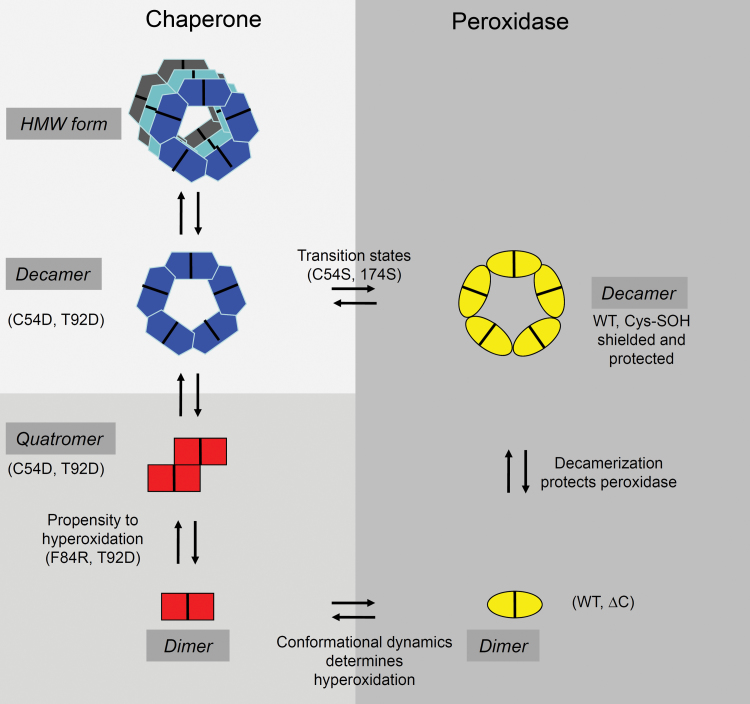
Fig. 9.
Summarizing scheme of the results and conclusions. 2-CysPrx adopts two principle different conformations, one with a peroxidase function and one with a chaperone function. The variants introduced in this study arrested certain conformations (C54S, C176S, C54D, C54DC176K, and F84R) and/or modified the flexibility of the protein (ΔC, C176S, and T92D). Hyperoxidation of C54 switches off the peroxidase and switched on the chaperone activity. However, hyperoxidation is not essential for chaperone activity (T92D, C54S, and C176S). This allowed us to suggest the function–structure relationships as indicated in the scheme. See text for further details. (This figure is available in colour at JXB online.)